Search Results
Showing results 1 to 20 of 23

Floating Paper Clip
Source Institutions
In this activity, challenge learners to float a paper clip in a cup of water. Learners discover that a paper clip will sink in a cup of water, except when it is placed on a piece of paper towel.

Oily Ice
Source Institutions
In this activity, learners experiment with the density of ice, water, and oil. Learners will discover that the density of a liquid determines whether it will float above or sink below another liquid.

From Gas to Liquid to Solid
Source Institutions
What causes frost to form on the outside of a cold container? In this activity, learners discover that liquid water can change states and freeze to become ice.

Fog Chamber
Source Institutions
In this weather-related activity, learners make a portable cloud in a bottle.

Having a Gas with Water
Source Institutions
In this activity, learners construct a simple electrolysis device. With this device, learners can decompose water into its elemental components: hydrogen and oxygen gas.

Drops on a Penny
Source Institutions
In this activity, challenge learners to predict and investigate how many water drops they can fit on one penny.

Indicating Electrolysis
Source Institutions
In this activity, learners build a simple electrolysis device. Then learners use an indicating solution to visualize hydrogen and oxygen molecules in water.

Mixing and Unmixing in the Kitchen
Source Institutions
In this chemistry investigation, learners combine common cooking substances (flour, baking powder, sugar, salt, pepper, oil, water, food coloring) to explore mixtures.

Molecules in Motion
Source Institutions
In this activity, learners add food coloring to hot and cold water to see whether heating or cooling affects the speed of water molecules.

The Ups and Downs of Thermometers
Source Institutions
In this activity, learners examine the parts of a thermometer. After placing a thermometer in hot and cold water, learners look at molecular model animations of the liquid in a thermometer.

Forgotten Genius
Source Institutions
This series of chemistry stations is designed to accompany the PBS documentary about African-American chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or p

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.

Crunch Time
Source Institutions
In this quick and easy activity and/or demonstration, learners use two empty 2-liter bottles and hot tap water to illustrate the effect of heat on pressure.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.

Gassy Lava Lamp
Source Institutions
In this activity, learners use oil, water, food coloring and antacid tablets to create a bubbling lava lamp. Use this activity to introduce concepts related to density, hydrophobicity vs.

Hollandaise Sauce: Emulsion at Work
Source Institutions
In this activity, learners follow a recipe to make hollandaise sauce. Learners discover how cooks use egg yolks to blend oil and water together into a smooth mix.

Why is the Sky Blue?
Source Institutions
In this activity, learners use a flashlight, a glass of water, and some milk to examine why the sky is blue and sunsets are red.
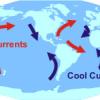
How it is Currently Done
Source Institutions
In this quick activity, learners observe how wind creates ocean currents.
Charge Challenge
Source Institutions
In this activity, learners explore how objects can have positive, negative, or neutral charges, which attract, repel and move between objects.
