Search Results
Showing results 1 to 20 of 88

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Changing the Density of a Liquid: Heating and Cooling
Source Institutions
Learners investigate how the temperature of water affects its density.

Heat Capacity: Can't Take the Heat?
Source Institutions
Why is ocean water sometimes the warmest when the average daily air temperature starts to drop? In this activity, learners explore the differing heat capacities of water and air using real data.

Supercooled Water Drops
Source Institutions
In this activity, learners touch supercooled water drops with an ice crystal and trigger the water drops to freeze instantly.

Make a Salt Volcano (Lava Lite)
Source Institutions
This activity about density provides instructions for making a miniature "lava lite" with just salt, oil, water, and food coloring.

Differing Densities: Fresh and Salt Water
Source Institutions
In this activity, learners visualize the differences in water density and relate this to the potential consequences of increased glacial melting.

Solar Water Heater
Learners work in teams to design and build solar water heating devices that mimic those used in residences to capture energy in the form of solar radiation and convert it to thermal energy.

Convection Current
Source Institutions
In this activity, learners make their own heat waves in an aquarium.

The Rain Man
Source Institutions
In this activity, learners observe the hydrologic cycle in action as water evaporates and condenses to form rain right before their eyes.
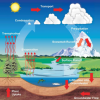
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.

Water Cycle in a Bag
Source Institutions
In this activity, learners create a biosphere in a baggie.

Leaf it to Me
Source Institutions
In this activity, learners observe the effect of transpiration as water is moved from the ground to the atmosphere.

From Gas to Liquid to Solid
Source Institutions
What causes frost to form on the outside of a cold container? In this activity, learners discover that liquid water can change states and freeze to become ice.

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.

Causes and Effects of Melting Ice
Source Institutions
In this activity, learners explore the concept of density-driven currents (thermohaline circulation) and how these currents are affected by climate change.

Why Doesn’t the Ocean Freeze?
Source Institutions
In this activity, learners explore how salt water freezes in comparison to fresh water.

Atmospheric Collisions
Source Institutions
In this activity/demonstration, learners observe what happens when two ping pong balls are suspended in the air by a hair dryer. Use this activity to demonstrate how rain drops grow by coalescence.

Evaporation
Source Institutions
This three-part activity consists of an activity that groups of learners develop themselves, a given procedure, and an optional demonstration.
Make Your Own Deep-Sea Vent
Source Institutions
In this activity, learners make a model of the hot water of a deep sea vent in the cold water of the ocean to learn about one of the ocean's most amazing and bizarre underwater habitats.
Let's Go Ice Fishing
Source Institutions
In this activity, learners are challenged to lift a floating ice cube out of a glass of water using just one end of a piece of string.
