Search Results
Showing results 1 to 20 of 49

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea
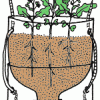
TerrAqua Investigation Column: What is the Land-Water Connection?
Source Institutions
In this investigation, learners plant seeds in a 2-liter bottle filled with soil that is connected to a water source below. Over the next few weeks, learners observe how the plants grow.

What's in the Water
Source Institutions
"What's in the Water" lets participants use tools to solve the mystery- what chemicals and compounds are in a sample of water?

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.
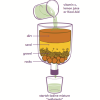
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Indicating Electrolysis
Source Institutions
Electrolysis is the breakdown of water into hydrogen and oxygen. This Exploratorium activity allows learners to visualize the process with an acid-based indicator.

What's Hiding in the Air?: Acid Rain Activity
As a model of acid rain, learners water plants with three different solutions: water only, vinegar only, vinegar-water mixture.

Diving Submarine
Source Institutions
Learners use a commercially available toy to experiment with density. They fill a chamber in the toy submarine with baking powder and release it into a tank of water.

Indicating Electrolysis
Source Institutions
In this activity, learners build a simple electrolysis device. Then learners use an indicating solution to visualize hydrogen and oxygen molecules in water.

Pop Rockets
Source Institutions
In this activity, learners make film canister rocket ships. A fin pattern is glued onto the outside of the canister, and fuel (water and half an antacid tablet) is mixed inside the canister.

Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.

Water Quality and pH Levels in Aquatic Ecosystems
Source Institutions
In this fun and in depth hands-on experiment, learners test various liquid samples (distilled water, lemon juice, vinegar, and baking soda mixed with water) to determine their pH levels and identify e

Forgotten Genius
Source Institutions
This series of chemistry stations is designed to accompany the PBS documentary about African-American chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or p

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

Hot Stuff!: Creating and Testing for Carbon Dioxide
In this demonstration, learners observe vinegar and baking soda reacting to form carbon dioxide (CO2) gas.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.

How Big is Small
Source Institutions
In this classic hands-on activity, learners estimate the length of a molecule by floating a fatty acid (oleic acid) on water.

Polishing Pennies
Source Institutions
In this experiment, learners try different liquids to see which ones clean pennies best. Liquids to try include water, lemon juice, cola, vinegar, and dishwashing detergent.
