Search Results
Showing results 1 to 8 of 8
Charge Challenge
Source Institutions
In this activity, learners explore how objects can have positive, negative, or neutral charges, which attract, repel and move between objects.

Kosher Dill Current: Make Your Own Battery!
Source Institutions
This is an activity that demonstrates how batteries work using simple household materials. Learners use a pickle, aluminum foil and a pencil to create an electrical circuit that powers a buzzer.
Penny for Your Thoughts
Source Institutions
In this activity, learners will explore how metals react with each other. They will see these metals change before their eyes as they coat a paperclip with the copper taken from a penny.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.

Dusted!
Source Institutions
Learners press their fingertip onto a clean Plexiglas sheet. The fingerprints are then revealed as learners dust over the print with fingerprint powder.

Change in Temperature: Exothermic Reaction
Source Institutions
Learners add calcium chloride to a baking soda solution and observe an increase in temperature along with the production of a gas and a white precipitate. These are all signs of a chemical reaction.
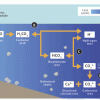
Coral, Carbon Dioxide and Calcification
Source Institutions
In this group activity, learners act out key stages of the "ocean carbon cycle" (also known as the "carbonate buffer system") through motions, rearranging blocks and team tasks.

Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.
