Search Results
Showing results 1 to 11 of 11

Metal Reactions
Source Institutions
This is written as a static display, but can easily be adapted to a hands-on experiment for learners to conduct.

Best Bubbles
Source Institutions
In this activity, learners experiment with creating various types of bubble solutions and testing which ingredients form longer-lasting bubbles.

Guar Gum Slime
Source Institutions
In this activity, learners create a gelatinous slime using guar gum powder and borax. Educators can use this simple activity to introduce learners to colloids.

Law of Conservation of Mass
Source Institutions
In this chemistry activity, learners explore whether matter is created or destroyed during a chemical reaction. They will compare the weight of various solutions before and after they are mixed.

Crystal Stencil Stars
Source Institutions
In this activity on page 6 of the PDF, learners dissolve Epsom salt in water and discover that the resulting solution can be used to create a work of art.
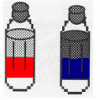
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

Exploring Size: Scented Solutions
Source Institutions
This is an activity in which learners will find that they can detect differences in concentration better with their nose (smelling) than with their eyes (seeing).

Double Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals to examine reactions that occur between two aqueous solutions.

Test Density with a Supersaturated Solution
Source Institutions
Learners create three solutions with different levels of salinity. They compare the density of these solutions by coloring them and layering them in a clear plastic cup and in a soda bottle.
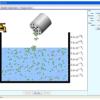
Salts & Solubility
Source Institutions
In this online interactive simulation, learners will add different salts to water and then watch the salts dissolve and achieve a dynamic equilibrium with solid precipitate.

Carrying Charges: Testing for Conductivity
Source Institutions
Learners are challenged to create solutions that conduct electricity and make a buzzer buzz (or an LED light up).
