Search Results
Showing results 61 to 80 of 153

Flubber
Source Institutions
Learners experiment with a piece of Silly Putty® by stretching, bouncing, and snapping it. They then create flubber, a similar substance, by mixing diluted glue and a solution of sodium borate.
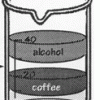
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Recycling Paper
Source Institutions
In this crafty chemistry activity (on page 2 of the PDF), learners make their own paper from used paper they may have otherwise thrown away.

Crocodiles
Source Institutions
Learners observe and compare the sizes of three toy “growing” crocodiles made from water-absorbent polymers. One is it its original state, dry, hard, and about 10cm long.

Electroplating
Source Institutions
In this activity, learners electrically plate zinc onto brass objects.

Resistance is Useful
Source Institutions
Learners write or draw with white crayon on white paper. They look and feel to detect their marks on the paper. Then, learners paint over their paper with watercolor paint.

Three Little Pigs
Source Institutions
In this activity, leaners explore building techniques by recreating the story of The Three Little Pigs.

Water Ways
Source Institutions
In this activity (on page 2 of the PDF), learners explore surface tension by adding pennies to cups which are "full" of plain water or soapy water.

Pearlescent Pigments
Source Institutions
This is written as a display, but can easily be adapted to a hands-on activity. Learners observe and shake containers of shiny liquids.

Iron in the Environment
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners corrode a penny in a cup with vinegar, salt water, and a source of iron (nails, paper clips, or twist ties).

Thar She Glows!
Source Institutions
Learners observe glow-in-the-dark objects in a homemade light-proof box. Objects can include glow sticks, glow-in-the-dark toys, and toys with fluorescent paint.

Swirling Milk
Source Institutions
In this chemistry activity, learners prepare two petri dishes, one filled with water and one filled with milk.

Burning Issues
Source Institutions
Learners use a candle to investigate the products of combustion. When a glass rod is held over a lit candle, the candle flame deposits carbon on the rod.

Lost Labels
Source Institutions
In this experiment, learners will conduct chemical and physical tests to identify mystery substances.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.

Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.

The Best Dam Simulation Ever
Source Institutions
This online simulation game explores the different consequences of water levels on the Columbia River in the Pacific Northwest.

Bridge the Gap
Source Institutions
Learners work in groups to construct bridges using stale marshmallows and toothpicks.

Designing Bandages
Source Institutions
Learners design different shaped bandages for different purposes. First, they draw their designs on paper.
Plastic Milk: You can make plastic from milk
Source Institutions
In this activity (on page 2 of the PDF), learners make a plastic protein polymer from milk. Adding vinegar to milk causes the protein casein to solidify or curdle.
