Search Results
Showing results 1 to 19 of 19

Metal Reactions
Source Institutions
This is written as a static display, but can easily be adapted to a hands-on experiment for learners to conduct.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.
Egg Osmosis
Source Institutions
Visitors observe three beakers. One beaker contains an egg immersed in vinegar. Visitors observe carbon dioxide gas escaping from the shell as the calcium carbonate reacts with the vinegar.

Silver Crystals
Source Institutions
This is written as a static display, but can easily become a hands-on experiment for learners.
Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

Starch Breakdown
Source Institutions
Learners use Benedict’s solution and heat to test for the presence of simple sugars in glucose, sucrose, starch, and starch combined with amylase.
Make Your Own DNA
Source Institutions
Learners match puzzle pieces to outlines of a DNA strand. The puzzle pieces represent the four chemicals making up DNA base pairs: adenine, thymine, guanine, and cytosine.

Starch Slime
Source Institutions
Learners mix liquid water with solid cornstarch. They investigate the slime produced, which has properties of both a solid and a liquid.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Crocodiles
Source Institutions
Learners observe and compare the sizes of three toy “growing” crocodiles made from water-absorbent polymers. One is it its original state, dry, hard, and about 10cm long.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.
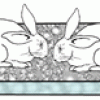
Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

Fireworks!
Source Institutions
In this chemistry lab activity, learners model the colors of fireworks by burning metallic solutions in a flame and observing the different colors produced.
First Impressions
Source Institutions
Learners experiment with a commercial photo-sensitive paper (Sunprint® or NaturePrint® paper). They place opaque and clear objects on the paper and expose it to bright light, observing the results.

Power To Go
Source Institutions
Learners observe an electrochemical cell constructed from a small jar containing zinc and copper strips immersed in separate solutions. The strips are connected to a motor that turns a small fan.

Starting Your Container Garden
Source Institutions
This guide outlines how to plant a garden even if you don't have a yard!

Phase Changes
Source Institutions
Learners observe a sealed test tube containing a small amount of solid stearic acid.

