Search Results
Showing results 61 to 80 of 86

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Currently Working
Source Institutions
Learners test solutions of water, sugar, salt, and hydrochloric acid for electrical conductivity. They immerse leads from a lighting device (a battery pack connected to an LED) into each solution.

Slide Rules
Source Institutions
Learners make their own simple slide rules out of paper and learn how they work.
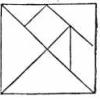
Tricky Tangrams
Source Institutions
In this activity (on pages 49-54 of PDF), learners play with tangrams, a set of triangles, squares and a parallelogram that can combine into a larger square as well as all sorts of other shapes.

Pot-in-Pot Refrigeration
Source Institutions
In this activity (on page 2 of PDF), learners create a low-tech refrigerator that requires no electricity to keep food from spoiling.

See the Light
Source Institutions
Learners mix a solution of luminol with hydrogen peroxide to produce a reaction that gives off blue light.

Bounce vs. Thud Balls
Source Institutions
Learners compare the properties of two balls that appear identical. One ball bounces, while the other ball "thuds." The “bounce” ball is made of the polymer polybutadiene (-C4H4-).

Fireworks!
Source Institutions
In this chemistry lab activity, learners model the colors of fireworks by burning metallic solutions in a flame and observing the different colors produced.

Jelly Beads
Source Institutions
Learners add drops of alginate solution to a solution of calcium chloride. The alginate does not mix with the calcium chloride, but forms soft gel beads.

Dusted!
Source Institutions
Learners press their fingertip onto a clean Plexiglas sheet. The fingerprints are then revealed as learners dust over the print with fingerprint powder.

Lava Lamps
Source Institutions
Learners observe working lava lamps to understand how they work (included in PDF link).

First Impressions
Source Institutions
Learners experiment with a commercial photo-sensitive paper (Sunprint® or NaturePrint® paper). They place opaque and clear objects on the paper and expose it to bright light, observing the results.

Human Battery
Source Institutions
Learners place their hands on different metals and use an ammeter to monitor the flow of electricity from one metal to another.
Bend It, Break It
Source Institutions
In this activity (on pages 25-32 of PDF), learners make models of the inner ear out of pipe cleaners.

Magical Möbius
Source Institutions
In this tabletop activity (on pages 32-40), learners make Möbius strips -- 3D surfaces with only one side.

How Loud is Too Loud
Source Institutions
In this activity (described on pages 39-42 of PDF), learners make a paper wheel (on pages 57-60 of PDF) that shows them the relative loudness of different sounds.

Electrolysis
Source Institutions
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water.

Paper Whites
Source Institutions
Learners observe different paper samples under ordinary room light and under a black light to learn some of the chemical differences between different types of paper.

Brick Drop Challenge
Source Institutions
In this design challenge, leaners attempt to build a strong structure out of LEGO® bricks that can withstand a 4-foot drop.

Power To Go
Source Institutions
Learners observe an electrochemical cell constructed from a small jar containing zinc and copper strips immersed in separate solutions. The strips are connected to a motor that turns a small fan.
