Search Results
Showing results 1 to 12 of 12

Luminol Test
Source Institutions
Learners mix a solution containing luminol and copper with a fake blood solution. A chemical reaction between the luminol solution and fake blood (hydrogen peroxide) show learners a blue glow.

Setting the Scene
Source Institutions
In this activity (on page 2), pairs of learners create an imaginary crime scene. One person leaves the room while the other person moves a few things around.

Common Scents
Source Institutions
Learners use a mortar and pestle to extract clove oil from cloves using denatured alcohol. They put this oil on paper, which they can take home.

What's Your Blood Type?
Source Institutions
In this activity, learners perform a simulated blood test procedure.

Concentrate!
Source Institutions
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution.

Acid Rain
Source Institutions
In this chemistry demonstration, acid rain is simulated in a petri dish.

Burning Issues
Source Institutions
Learners use a candle to investigate the products of combustion. When a glass rod is held over a lit candle, the candle flame deposits carbon on the rod.

Rock Bottoms
Source Institutions
Learners add acid rain (nitric acid) to two cups that represent lakes. One cup contains limestone gravel and the other contains granite gravel.
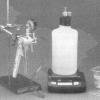
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Jelly Beads
Source Institutions
Learners add drops of alginate solution to a solution of calcium chloride. The alginate does not mix with the calcium chloride, but forms soft gel beads.

As The Stomach Churns
Source Institutions
In this chemistry activity, learners fill two test tubes with a solution of "artificial stomach fluid," consisting of hydrochloric acid in the same concentration as in human stomachs, some soap to cre
