Search Results
Showing results 1 to 17 of 17

Metal Reactions
Source Institutions
This is written as a static display, but can easily be adapted to a hands-on experiment for learners to conduct.

Mystery Powders
Source Institutions
Learners are given mysterious white powders and have to determine their identity with chemical tests.

Luminol Test
Source Institutions
Learners mix a solution containing luminol and copper with a fake blood solution. A chemical reaction between the luminol solution and fake blood (hydrogen peroxide) show learners a blue glow.

Divers
Source Institutions
Learners experiment with a 2-liter plastic bottle containing water and four “divers." The divers consist of open, transparent containers with the opening points downward.

Twisted Tesselations
Source Institutions
In this activity (on pages 41-47 of PDF), learners explore tesselating geometric patterns (repeated shapes, similar to the art of M.C. Escher).

Trading Places
Source Institutions
In this activity, learners discover that atoms and ions of different metals will change places.

What's Your Blood Type?
Source Institutions
In this activity, learners perform a simulated blood test procedure.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).

Acid Rain
Source Institutions
In this chemistry demonstration, acid rain is simulated in a petri dish.

Burning Issues
Source Institutions
Learners use a candle to investigate the products of combustion. When a glass rod is held over a lit candle, the candle flame deposits carbon on the rod.

Rock Bottoms
Source Institutions
Learners add acid rain (nitric acid) to two cups that represent lakes. One cup contains limestone gravel and the other contains granite gravel.

Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.
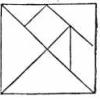
Tricky Tangrams
Source Institutions
In this activity (on pages 49-54 of PDF), learners play with tangrams, a set of triangles, squares and a parallelogram that can combine into a larger square as well as all sorts of other shapes.

See the Light
Source Institutions
Learners mix a solution of luminol with hydrogen peroxide to produce a reaction that gives off blue light.

Power To Go
Source Institutions
Learners observe an electrochemical cell constructed from a small jar containing zinc and copper strips immersed in separate solutions. The strips are connected to a motor that turns a small fan.

