Search Results
Showing results 1 to 20 of 20
Trading Places: Redox Reactions
Source Institutions
Visitors add drops of copper sulfate solution onto a steel nail. They observe the nail change color from silver to brown as the copper plates onto the nail.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.

DNA Nanotechnology
Source Institutions
In this activity, learners explore deoxyribonucleic acid (DNA), a nanoscale structure that occurs in nature.

Egg-Citing Physics
Source Institutions
In this demonstration about momentum, use physics to distinguish between a hard-boiled egg and a raw egg without cracking them open.

Model Wind Tunnel
Source Institutions
In this activity, learners build a miniature wind tunnel to measure force. Learners construct the model out of Lexan plastic, a fan, and a precise digital scale.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Laser Projection Microscope
Source Institutions
In this activity, learners use a laser pointer to project a microscopic image of a liquid sample suspended from the tip of a syringe.
Concentrate: Concentrations and Reaction Rates
Source Institutions
Visitors incrementally increase the amount of iodate in three different test tubes containing the same amount of a starch solution.
Mystery Tubes
Source Institutions
Learners investigate a pre-constructed mystery tube to determine its interior mechanism.

Creating a Local Field Guide
Source Institutions
In this activity, learners survey living organisms near where they live or go to school, and create a local field guide.

Currently Working
Source Institutions
Learners test solutions of water, sugar, salt, and hydrochloric acid for electrical conductivity. They immerse leads from a lighting device (a battery pack connected to an LED) into each solution.

Clam Hooping
Source Institutions
In this two-part outdoor activity, learners conduct a population census of squirting clams on a beach or mudflat, and investigate the clams' natural history.

Wild Sourdough
Source Institutions
In this activity, learners explore chemistry and the microbial world by making their own sourdough starter and bread at home using only flour and water.

Forgotten Genius
Source Institutions
This series of chemistry stations is designed to accompany the PBS documentary about African-American chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or p

Fish Features and Habitats
Source Institutions
In this activity, learners observe live fish in tanks to consider how their body structures are related to their behaviors and habitats.
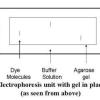
Gel Electrophoresis of Dyes
Source Institutions
In this experiment related to plant biotechnology, learners discover how to prepare and load an electrophoresis gel.
