Search Results
Showing results 1 to 20 of 20

Formation of a Precipitate
Source Institutions
Learners create hard water by mixing Epsom salt and water. Then they compare what happens when soap solution is mixed with hard water and regular water.
Gas Production: Blow up a balloon!
Source Institutions
In this classic reaction, learners baking soda and vinegar in a soda bottle to produce carbon dioxide (CO2) gas. This gas inflates a balloon.

Ready, Set, Fizz!
Source Institutions
In this activity, learners explore the chemical reaction between water and effervescent antacid tablets. This hands-on activity models how a material can act differently when it's nanometer-sized.

Using Chemical Change to Identify an Unknown
Source Institutions
In this activity, learners will develop a method to test five similar-looking powders (baking soda, baking powder, cream of tartar, detergent, and cornstarch) with four test liquids (water, vinegar, i
Rockets Away
Source Institutions
In this activity, learners build a simple "rocket" with ordinary household materials to demonstrate the basic principles behind rocketry and the principle of reaction.

Polishing Pennies
Source Institutions
In this experiment, learners try different liquids to see which ones clean pennies best. Liquids to try include water, lemon juice, cola, vinegar, and dishwashing detergent.

Powder Particulars
Source Institutions
In this introductory activity and demonstration, learners are introduced to the concept that different substances react chemically in characteristic ways.

DIY Elephant Toothpaste
Source Institutions
In this activity, learners will experiment with catalysts to create an at-home version of elephant toothpaste.
Matter of Degree
Source Institutions
In two separate bags, learners mix water with Epsom salts and detergent.

Breaking Up with Combustion
Source Institutions
This activity teaches combustion as the interaction of a fuel source and oxygen.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.

Operation Espionage
Source Institutions
In this activity, learners create and reveal secret messages written with invisible ink! The invisible ink is actually a baking soda solution, and the magical revealing liquid is fruit juice.

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.

DIY Bath Bombs
Source Institutions
In this activity, learners will explore acid-base reactions and create their own bubbly results.

Diving Submarine
Source Institutions
Learners use a commercially available toy to experiment with density. They fill a chamber in the toy submarine with baking powder and release it into a tank of water.

Flubber: Make a polymer!
Source Institutions
This activity (on page 2 of the PDF) features a recipe to create the stretchy polymer Flubber from Borax detergent, white glue, and water.

Create Gas
Source Institutions
Learners mix vinegar and baking soda together in a bottle to create a chemical reaction. The reaction produces a gas, carbon dioxide, which inflates a balloon attached to the mouth of the bottle.

Snowstorm in a Jar
Source Institutions
In this activity, learners will experiment with density and chemical reactions to create a flurry activity.
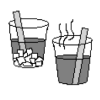
Glow Fast, Glow Slow: Alter the Rate of a Reaction!
Source Institutions
Learners investigate one factor affecting reaction rates: temperature. In a darkened room, two identical lightsticks are placed in water -- one in hot water and one in cold water.

Exploring Properties: Surface Area
Source Institutions
This hands-on activity demonstrates how a material can act differently when it's nanometer-sized.
