Search Results
Showing results 1 to 9 of 9

Dunking the Planets
Source Institutions
In this demonstration, learners compare the relative sizes and masses of scale models of the planets as represented by fruits and other foods.

Burn a Peanut
Source Institutions
In this activity, learners burn a peanut, which produces a flame that can be used to boil away water and count the calories contained in the peanut.

Space Jell-O
Source Institutions
Albert Einstein proved that space bends around anything that has mass. This activity uses Jell-O's ability to bend around objects as a model for space bending around planets and stars.

Comparing the Density of Different Liquids
Source Institutions
Learners carefully pour vegetable oil, water, and corn syrup in any order into a cup and discover that regardless of the order they are poured, the liquids arrange themselves in layers the same way.

Get the Porridge Just Right
Source Institutions
Learners set up three different bowls, each with a different mass of oatmeal. Learners monitor the temperature of the oatmeal and find that larger masses take longer to cool.

Wrap It Up!
Source Institutions
In this Energy and Environment activity (page 9 of the PDF), learners calculate the mass of a piece of gum, compare it to the mass of the gum's packaging, and then create a bar graph of the results.

Having a Gas with Cola
Source Institutions
In this activity, learners measure the amount of carbon dioxide in a carbonated drink.

Ship the Chip
Source Institutions
In this activity, learners explore engineering package designs that meet the needs of safely shipping a product.
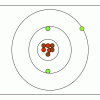
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).
