Search Results
Showing results 1 to 11 of 11

Metal Reactions
Source Institutions
This is written as a static display, but can easily be adapted to a hands-on experiment for learners to conduct.

Divers
Source Institutions
Learners experiment with a 2-liter plastic bottle containing water and four “divers." The divers consist of open, transparent containers with the opening points downward.
Floating Golf Ball
Source Institutions
Visitors observe a graduated cylinder with a golf ball floating about halfway in liquid. The bottom half of the cylinder contains a concentrated solution of salt.
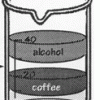
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Swirling Milk
Source Institutions
In this chemistry activity, learners prepare two petri dishes, one filled with water and one filled with milk.

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.

Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

See the Light
Source Institutions
Learners mix a solution of luminol with hydrogen peroxide to produce a reaction that gives off blue light.

Shake It Up!
Source Institutions
Learners observe a sealed container holding a clear colorless liquid. They shake the container and the fluid turns blue. When allowed to sit for a few moments, the fluid turns colorless again.

Power To Go
Source Institutions
Learners observe an electrochemical cell constructed from a small jar containing zinc and copper strips immersed in separate solutions. The strips are connected to a motor that turns a small fan.

