Search Results
Showing results 1 to 14 of 14
Trading Places: Redox Reactions
Source Institutions
Visitors add drops of copper sulfate solution onto a steel nail. They observe the nail change color from silver to brown as the copper plates onto the nail.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.

Copper Cleanup
Source Institutions
In this hands-on experiment, kids use chemistry to explore whether acids or bases are better at restoring a penny’s shine.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.
Concentrate: Concentrations and Reaction Rates
Source Institutions
Visitors incrementally increase the amount of iodate in three different test tubes containing the same amount of a starch solution.

Sidewalk Chalk
Source Institutions
In this chemistry activity, learners witness an exothermic reaction, while making their very own, completely usable sidewalk chalk. This is also an excellent activity for exploring color mixing.

Tempest in a Teacup
Source Institutions
In this hands-on activity, learners determine the types of chemical reactions achieved when combining different household products.
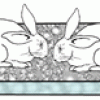
Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

Floating Dry Erase Creations
Source Institutions
In this activity, learners will create a drawing with dry erase markers and watch it come to life. Learners will explore chemistry, art and storytelling through this activity.

Chemistry Cake
Source Institutions
In this exciting and tasty chemistry activity which requires adult supervision, learners explore how chemistry affects a simple everyday activity like cooking.
