Search Results
Showing results 1 to 20 of 27

Tiny Tubes
Source Institutions
In this activity, learners make "totally tubular" forms of carbon. Learners use chicken wire to build macro models of carbon nanotubes.

Smelly Balloons
Source Institutions
In this activity, learners sniff out scents hidden in balloons! After investigating, learners discover we sometimes can use another sense (smell) to detect things too small to see.
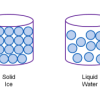
Atoms and Matter (3-6)
Source Institutions
In this activity, learners build models of atoms and molecules, then consider their role in different phases of matter, density, and mixtures and solutions.

Odors Aloft
Source Institutions
Learners smell balloons filled with different scents to guess what's inside. From this, they infer the presence and motion of scented molecules.

Trading Places
Source Institutions
In this activity, learners discover that atoms and ions of different metals will change places.

Dusting For Fingerprints
Source Institutions
In this activity, learners become detectives and use chemistry to investigate fingerprints.

Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

What's So Special about Water: Surface Tension
Source Institutions
In this three-part activity, learners play a game and conduct two simple experiments to explore water and surface tension. Learners will have fun discovering how water "sticks" together.

What's So Special about Water: Solubility and Density
Source Institutions
In this activity about water solubility and density, learners use critical thinking skills to determine why water can dissolve some things and not others.

Four of the States of Matter
Source Institutions
This kinesthetic science demonstration introduces learners to four states of matter: solid, liquid, gas, and plasma.
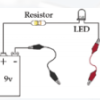
Exploring Materials: Graphene
Source Institutions
In this activity on page 4 of the PDF, explore the unique molecular structure and conductive nature of graphene. Learners construct a circuit with a battery and LED bulb.

Stick to It: Adhesion II
Source Institutions
Water sticks to all kinds of things in nature — flowers, leaves, spider webs - and doesn't stick to others, such as a duck's back.

Thar She Glows!
Source Institutions
Learners observe glow-in-the-dark objects in a homemade light-proof box. Objects can include glow sticks, glow-in-the-dark toys, and toys with fluorescent paint.
Glitter Globe
Source Institutions
This activity shows learners (with adult supervision) how to make a Glitter Globe, a fabulous toy that shimmers when you shake it.

Rutherford Roller
Source Institutions
In this activity, learners make a black box device that serves as an excellent analogy to Rutherford's famous experiment in which he deduced the existence of the atomic nucleus.

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.

Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.

Levity Through Tension: Fun with Water's Surface Tension
Source Institutions
This experiment describes how to create a "dribble bottle" which only leaks water when the cap is unscrewed. The full water bottle has a small hole made with a push pin.

Nuclear Fusion
Source Institutions
This simple and engaging astronomy activity explains nuclear fusion and how radiation is generated by stars, using marshmallows as a model.

Molecules in Motion
Source Institutions
"Molecules in Motion" explores how materials behave and change in a vacuum.
