Search Results
Showing results 1 to 9 of 9

Sweetly Balanced Equations
Source Institutions
In this (edible) activity, learners balance chemical equations using different kinds and colors of candy that represent different atoms. Learners will work in pairs and explore conservation of atoms.

Mysterious M&M's
Source Institutions
Learners place an M&M candy in water and observe what happens. The sugar-and-color coating dissolves and spreads out in a circular pattern around the M&M.

Racing M&M Colors
Source Institutions
Learners design their own experiment to determine which M&M color dissolves the fastest in water.

Edible Glass
Source Institutions
In this activity, learners discover the principles of edible glass by making a supersaturated sugar solution.

Canned Heat
Source Institutions
In this activity, learners explore how light and dark colored objects absorb the Sun's radiations at different rates.

Diffusion of Water with Gummy Bears
Source Institutions
In this activity, learners investigate the movement of water into and out of a polymer. Learners test the diffusion of water through gummy bears, which are made of sugar and gelatin (a polymer).

Wrap It Up!
Source Institutions
In this Energy and Environment activity (page 9 of the PDF), learners calculate the mass of a piece of gum, compare it to the mass of the gum's packaging, and then create a bar graph of the results.

Demonstrating An Epidemic
Source Institutions
This experiment allows learners to experience a small scale "epidemic," demonstrating the ease with which disease organisms are spread, and enables learners to determine the originator of the "epidemi
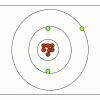
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).
