Search Results
Showing results 21 to 31 of 31
Coral and Chemistry
Source Institutions
In this experiment, learners will explore whether increased carbon dioxide makes our oceans more basic or more acidic.

Film Canister Rocket
Source Institutions
In this activity, learners construct and launch rockets using simple materials and their understanding of chemical reactions.

Avogadro's Bubbly Adventure
Source Institutions
In this activity on page 7 of the PDF, learners investigate the solubility of gas in water at different temperatures. This experiment will help learners determine if temperature affects solubility.

Do Plants Need Sunlight?
Source Institutions
In this activity, learners find out what happens when they cover leaves with pieces of black construction paper. This activity shows learners that plants need sunlight to survive.

Production of a Gas: Controlling a Chemical Reaction
Source Institutions
Learners mix vinegar and baking soda to produce a gas. With the addition of a bit of liquid soap, the gas becomes trapped in measurable bubbles.

A Mole of Gas
Source Institutions
In this two-part activity, learners use everyday materials to visualize one mole of gas or 22.4 liters of gas. The first activity involves sublimating dry ice in large garbage bag.

Shell Shifts
Source Institutions
Ocean acidification is a big issue due to the amount of carbon dioxide humans release. CO2 in the atmosphere is absorbed into the ocean thus changing its acidity.
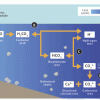
Coral, Carbon Dioxide and Calcification
Source Institutions
In this group activity, learners act out key stages of the "ocean carbon cycle" (also known as the "carbonate buffer system") through motions, rearranging blocks and team tasks.

Create Gas
Source Institutions
Learners mix vinegar and baking soda together in a bottle to create a chemical reaction. The reaction produces a gas, carbon dioxide, which inflates a balloon attached to the mouth of the bottle.

The Carbon Cycle and its Role in Climate Change: Activity 3
Source Institutions
In this activity, learners explore the human influences on the carbon cycle and examine how fossil fuels release carbon.

Having a Gas with Cola
Source Institutions
In this activity, learners measure the amount of carbon dioxide in a carbonated drink.
