Search Results
Showing results 1 to 20 of 26

Cleaning with Dirt
Source Institutions
Learners build a filter from old soda bottles and dirt. They create polluted water, and pour it through their filter to clean it.

Latent Prints
Source Institutions
In this activity, learners examine fingerprints. Learners leave a hidden print on a surface and then make their own print detecting powder from graphite (found in pencils).

Chromatography Can Separate!
Source Institutions
In this chemistry activity, learners use thin layer chromatography to determine the molecular composition of different markers.
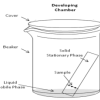
Separating with Chromatography
Source Institutions
In this experiment, learners separate different types of molecules in marker inks (using a technique called "thin layer chromatography").
Atoms and Matter (3-6)
Source Institutions
In this activity, learners build models of atoms and molecules, then consider their role in different phases of matter, density, and mixtures and solutions.

Kool Colors
Source Institutions
Learners investigate how temperature affects the rate of chemical reactions by observing how steel wool reacts with various types of Kool-Aid solutions at different temperatures.

Odors Aloft
Source Institutions
Learners smell balloons filled with different scents to guess what's inside. From this, they infer the presence and motion of scented molecules.

Density Rainbow
Source Institutions
In this activity, learners mix several sugar solutions to investigate the property of density. Each sugar solution has a different density and color of the rainbow.

Color Me Blue
Source Institutions
In this activity, learners add dilute bleach solution to water that has been dyed with yellow, blue, and green food color.

Of Cabbages and Kings
Source Institutions
This lesson gives full instructions for making cabbage juice indicator, a procedure sheet for learners to record observations as they use the indicator to test materials, and extension activities to d
Penny for Your Thoughts
Source Institutions
In this activity, learners will explore how metals react with each other. They will see these metals change before their eyes as they coat a paperclip with the copper taken from a penny.

Matter of Degree
Source Institutions
In two separate bags, learners mix water with Epsom salts and detergent.

Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

Oil Spill Solutions
Source Institutions
In this activity, learners explore how environmental engineers might approach solving the problem of an oil spill.

Lost Labels
Source Institutions
In this experiment, learners will conduct chemical and physical tests to identify mystery substances.
Coral and Chemistry
Source Institutions
In this experiment, learners will explore whether increased carbon dioxide makes our oceans more basic or more acidic.

Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.

Big Things Come in Little Packages
Source Institutions
As a group, learners investigate three packages which are all the same size and shape, but have different contents. One is filled with foam, one is filled with wood, and one is filled with metal.
Choose Your Ooze
Source Institutions
During this activity, learners will make different versions of "ooze" using varied proportions of detergent and glue.

Pollution Diffusion
Source Institutions
Learners design their own experiment to investigate how pollution diffuses through ground material.
