Search Results
Showing results 1 to 20 of 24
The Shape of Floatation
Source Institutions
In this activity (on page 2 of the PDF under GPS: Sailboat Design Activity), learners will discover how the shape of an object, not just its weight, determines whether it floats or sinks.

Comparing the Density of an Object to the Density of Water
Source Institutions
Learners compare the weight of equal volumes of wax, water, and clay. Learners discover that since the wax weighs less than an equal volume of water, it is less dense than water and will float.

Defining Density
Source Institutions
In this introductory demonstration and activity, learners are introduced to the concept of density as they explore a rock and a wooden block in water.

Changing the Density of an Object: Changing Shape
Source Institutions
Learners will see that changing the shape of an object, like a clay ball, that is more dense than water, can affect whether the object will sink or float.

Diet Light
Source Institutions
In this quick activity, learners observe how the added sugar in a can of soda affects its density and thus, its ability to float in water.

Descartes' Diver
Source Institutions
In this activity, learners explore how changes in fluid pressure affect the buoyancy of a Cartesian diver inside a plastic soda bottle.

Density Rainbow
Source Institutions
In this activity, learners mix several sugar solutions to investigate the property of density. Each sugar solution has a different density and color of the rainbow.

Above Water: Buoyancy & Displacement
Source Institutions
In an investigation called "Shape It!" learners craft tiny boats out of clay, set them afloat on water and then add weight loads to them, in order to explore: how objects stay afloat in water; what th

Using Color to See How Liquids Combine
Source Institutions
Learners add different liquids (water, salt water, alcohol, and detergent solution) to water and observe the different ways the different liquids combine with water.

Hovercraft
Source Institutions
In this activity, learners build a hovercraft using a paper plate, cup, and simple motor.

Floating Head Cup
Source Institutions
In this activity, learners watch a figure "magically" float up through the air.

Uplifting Force: Buoyancy & Density
Source Institutions
In this investigation, learners explore the force known as buoyancy by placing various objects into water and observing how they behave (for example, which sink more quickly, which float, how much wat

Dancing Spaghetti
Source Institutions
In this chemistry activity, learners use spaghetti to explore density and chemical reactions.

Below the Surface: Surface Tension II
Source Institutions
In this activity learners explore surface tension. Why are certain objects able to float on the surface of water and how do detergents break the surface tension of water?
Boats Afloat
Source Institutions
In this activity, learners discover what buoyancy is and determine the characteristics that make an object buoyant. Learners design, build, test, and evaluate boats made from a variety of materials.

Changing the Density of an Object: Adding Material
Source Institutions
Learners see that a can of regular cola sinks while a can of diet cola floats. As a demonstration, bubble wrap is taped to the can of regular cola to make it float.
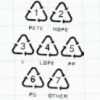
Sink or Swim?
Source Institutions
In this activity, learners identify different plastics in a mystery bag. Learners discover that plastics are classified #1 through #7.

Color Splash
Source Institutions
In this activity, learners mix water, cooking oil, and liquid food coloring to create beautiful colored designs in a cup. Use this activity to explore liquid density and solubility.
Watercraft
Source Institutions
In this design challenge activity, learners build a boat that can hold 25 pennies (or 15 one inch metal washers) for at least ten seconds before sinking.
Milli's Super Sorting Challenge
Source Institutions
In this activity, learners separate materials based on their special properties to mimic the way recyclables are sorted at recycling centers.
