Search Results
Showing results 1 to 12 of 12

Mystery Powders
Source Institutions
Learners are given mysterious white powders and have to determine their identity with chemical tests.

Gas Production: Blow up a balloon!
Source Institutions
In this classic reaction, learners baking soda and vinegar in a soda bottle to produce carbon dioxide (CO2) gas. This gas inflates a balloon.

Odors Aloft
Source Institutions
Learners smell balloons filled with different scents to guess what's inside. From this, they infer the presence and motion of scented molecules.

Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

Balloon in a Flask
Source Institutions
Learners observe a flask with a balloon attached over the mouth and inverted inside the flask.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.
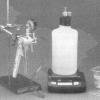
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.

Pot-in-Pot Refrigeration
Source Institutions
In this activity (on page 2 of PDF), learners create a low-tech refrigerator that requires no electricity to keep food from spoiling.

Diving Submarine
Source Institutions
Learners use a commercially available toy to experiment with density. They fill a chamber in the toy submarine with baking powder and release it into a tank of water.

Shake It Up!
Source Institutions
Learners observe a sealed container holding a clear colorless liquid. They shake the container and the fluid turns blue. When allowed to sit for a few moments, the fluid turns colorless again.

Yeast Balloons: Can biochemistry blow up a balloon?
Source Institutions
Using yeast, sugar, and water, learners create a chemical reaction which produces carbon dioxide (CO2) gas inside a 2-liter bottle. They use this gas to inflate a balloon.

As The Stomach Churns
Source Institutions
In this chemistry activity, learners fill two test tubes with a solution of "artificial stomach fluid," consisting of hydrochloric acid in the same concentration as in human stomachs, some soap to cre
