Search Results
Showing results 1 to 7 of 7

Fast Rusting
Source Institutions
In this activity, learners conduct an experiment to find out if steel wool will weigh more or less when it is burned. Learners will explore the effects of oxidation and rusting on the steel wool.
Properties of Metals
Source Institutions
In this activity, learners explore the properties of metals at four stations. The stations include A) Magnetism and Breakfast Cereal; B) Conductivity of Metals; C) Alloys; and D) Metal Plating.
Penny for Your Thoughts
Source Institutions
In this activity, learners will explore how metals react with each other. They will see these metals change before their eyes as they coat a paperclip with the copper taken from a penny.

Combustion
Source Institutions
In this chemistry activity, learners discover that the weight of the product of combustion is greater than that of the starting material.

Iron in Cereal: Find iron in your food!
Source Institutions
Learners investigate an iron-fortified cereal by stirring it with a strong magnet. They discover that metallic iron is present in some cereals.
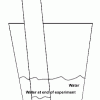
Percentage of Oxygen in the Air
Source Institutions
In this activity, learners calculate the percentage of oxygen in the atmosphere by using steel wool's ability to rust.

Iron for Breakfast
Source Institutions
Did you know that some breakfast cereals are fortified with ferric phosphate, while others contain tiny pieces of reduced iron?
