Search Results
Showing results 1 to 10 of 10

Underwater Fireworks
Source Institutions
In this activity, learners investigate diffusion by creating underwater "fireworks" using food coloring, oil and water.

Red, White and Blue II Demonstration
Source Institutions
In this chemistry demonstration, learners investigate the rule "likes dissolve likes" by combining three, immiscible liquids to create a colorful density column.

Molecules in Motion
Source Institutions
In this activity, learners add food coloring to hot and cold water to see whether heating or cooling affects the speed of water molecules.

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.
Daffy Density
Source Institutions
In this chemistry activity, learners explore density by using four solids and 6 liquids to create colorful, layered rows.

The Ups and Downs of Thermometers
Source Institutions
In this activity, learners examine the parts of a thermometer. After placing a thermometer in hot and cold water, learners look at molecular model animations of the liquid in a thermometer.
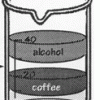
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Energetic Water
Source Institutions
In this activity, learners explore how hot and cold water move. Learners observe that temperature and density affect how liquids rise and fall.

Test Density with a Supersaturated Solution
Source Institutions
Learners create three solutions with different levels of salinity. They compare the density of these solutions by coloring them and layering them in a clear plastic cup and in a soda bottle.

Oily Ice
Source Institutions
In this activity, learners experiment with the density of ice, water, and oil. Learners will discover that the density of a liquid determines whether it will float above or sink below another liquid.
