Search Results
Showing results 1 to 17 of 17

Odors Aloft
Source Institutions
Learners smell balloons filled with different scents to guess what's inside. From this, they infer the presence and motion of scented molecules.

Sweetly Balanced Equations
Source Institutions
In this (edible) activity, learners balance chemical equations using different kinds and colors of candy that represent different atoms. Learners will work in pairs and explore conservation of atoms.
Properties of Metals
Source Institutions
In this activity, learners explore the properties of metals at four stations. The stations include A) Magnetism and Breakfast Cereal; B) Conductivity of Metals; C) Alloys; and D) Metal Plating.
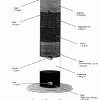
Daffy Density
Source Institutions
In this chemistry activity, learners explore density by using four solids and 6 liquids to create colorful, layered rows.

Conservation of Mass
Source Institutions
This activity was designed for blind learners, but all types of learners can participate to learn about conservation of gas. This is one of the classic experiments using baking soda and vinegar.

Starch Slime
Source Institutions
Learners mix liquid water with solid cornstarch. They investigate the slime produced, which has properties of both a solid and a liquid.

Air, It's Really There
Source Institutions
This lesson focuses on molecular motion in gases. Learners compare the mass of a basketball when it is deflated and after it has been inflated.

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.

Big Things Come in Little Packages
Source Institutions
As a group, learners investigate three packages which are all the same size and shape, but have different contents. One is filled with foam, one is filled with wood, and one is filled with metal.

Squidgy Slime
Source Institutions
In this chemistry activity, learners transform two ingredients (4% polyvinyl alcohol solution and 4% borax solution) into gooey slime.
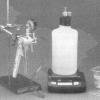
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.
Milli's Super Sorting Challenge
Source Institutions
In this activity, learners separate materials based on their special properties to mimic the way recyclables are sorted at recycling centers.

Chemical Change
Source Institutions
In this chemistry activity, learners explore the amount of copper in a new penny. Learners use toilet bowl cleaner to hollow out the interior of a penny with zinc inside.

Sublimation Bubbles
Source Institutions
"Sublimation Bubbles" allows learners to explore how some solid materials, such as dry ice, can phase change directly from their solid to gaseous form.

Floating Dry Erase Creations
Source Institutions
In this activity, learners will create a drawing with dry erase markers and watch it come to life. Learners will explore chemistry, art and storytelling through this activity.

What's the Matter
Source Institutions
In this activity, learners identify different classes of matter based on physical properties.
