Search Results
Showing results 1 to 20 of 21

Smelly Balloons
Source Institutions
In this activity, learners sniff out scents hidden in balloons! After investigating, learners discover we sometimes can use another sense (smell) to detect things too small to see.

Surface Tension Icebreaker
Source Institutions
This is a quick activity (located on page 2 of the PDF under Nasturtium Leaves Activity) about surface tension.

Surface Area and Soda Geysers
Source Institutions
This is an activity (located on page 4 of the PDF under Surface Area Activity) about surface area and reactivity.

Iridescent Art
Source Institutions
This is a quick activity (on page 2 of the PDF under Butterfly Wings Activity) that illustrates how nanoscale structures, so small they're practically invisible, can produce visible/colorful effects.

Make a Human Scale Ladder
Source Institutions
In this quick activity about size and scale (on page 2 of the PDF under What's Nano?
No bones about it!
Source Institutions
This is an activity (located on page 3 of the PDF) about the mixture of materials in bone and how they affect its strength.

Lotus Leaf Effect
Source Institutions
This is a demonstration about how nature inspires nanotechnology. It is easily adapted into a hands-on activity for an individual or groups.
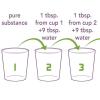
Sniffing for a Billionth
Source Institutions
This is an activity (located on page 4 of the PDF under What's Nano? Activity) about size and scale.

Space Elevator
Source Institutions
In this activity, learners imagine what the world might look like if we could build an elevator to space!

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.

Bridge Building
Source Institutions
This is a quick activity (on page 2 of the PDF under Hockey Sticks Activity) about how the arrangement of carbon atoms determines carbon's different properties.

Science of Sunblock
Source Institutions
This is an activity (located on page 3 of the PDF under Stained Glass Activity) about nanotechnology making its way into everyday products, such as sunscreen, and how effective these invisible particl

Smelly Balloons
Source Institutions
Are balloons porous or non-porous? In this activity, learners watch an entertaining Mr. O video and conduct a simple experiment to find out.

Self-Assembly Game
Source Institutions
This is a quick game about self-assembly (page 2 of PDF under Self-Assembly Activity). Like the molecules of DNA, learners will self-assemble into a pattern by following a simple set of rules.

Model Well
Source Institutions
In this quick activity about pollutants and groundwater (page 2 of PDF under Water Clean-up Activity), learners build a model well with a toilet paper tube.

Moving Molecules!
Source Institutions
In this activity about molecular diffusion (located on page 2 of the PDF under Nanosilver Activity), learners will make predictions and move molecules of iodine through a seemingly solid plastic sandw

Gecko Feet
Source Institutions
This is an activity (located on page 3 of PDF under Gecko Feet Activity) about modeling a nanoscale phenomenon (gravity-defying gecko feet) with macroscale objects (shoes).
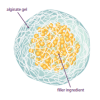
Self-Assembling Dessert Toppings
Source Institutions
This is an activity (located on page 3 of the PDF under Self-Assembly Activity) about self-assembly, the ability of molecules to assemble themselves according to certain rules.
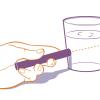
Beam Me Up!
Source Institutions
This is a quick activity (on page 2 of the PDF under Stained Glass Activity) about the "Tyndall effect," the scattering of visible light when it hits very small dispersed particles.

Shrinking Cups
Source Institutions
This is a quick activity (on page 2 of the PDF under Gecko Feet Activity) about the forces of gravity and surface tension and how their behavior is influenced by size.
