Search Results
Showing results 1 to 13 of 13

Acid (and Base) Rainbows
Learners use red cabbage juice and pH indicator paper to test the acidity and basicity of household materials. The activity links this concept of acids and bases to acid rain and other pollutants.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

Jell-O Model of Microfluidics
Source Institutions
This activity uses Jell-O(R) to introduce learners to microfluidics, the flow of fluids through microscopic channels.

Acid Rain Effects
Learners conduct a simple experiment to model and explore the harmful effects of acid rain (vinegar) on living (green leaf and eggshell) and non-living (paper clip) objects.

Hot Stuff!: Testing for Carbon Dioxide from Our Own Breath
Learners blow into balloons and collect their breath--carbon dioxide gas (CO2). They then blow the CO2 from the balloon into a solution of acid-base indicator.

Hot Stuff!: Creating and Testing for Carbon Dioxide
In this demonstration, learners observe vinegar and baking soda reacting to form carbon dioxide (CO2) gas.

Indicating Electrolysis
Source Institutions
Electrolysis is the breakdown of water into hydrogen and oxygen. This Exploratorium activity allows learners to visualize the process with an acid-based indicator.

Hot Stuff!: Testing Ice
In this demonstration, learners compare and contrast regular water ice to dry ice (frozen carbon dioxide). Both samples are placed in a solution of acid-base indicator.

pH Scale
Source Institutions
In this online interactive simulation, learners will test the pH of liquids like coffee, spit, and soap to determine whether each is acidic, basic, or neutral.
Plastic Milk: You can make plastic from milk
Source Institutions
In this activity (on page 2 of the PDF), learners make a plastic protein polymer from milk. Adding vinegar to milk causes the protein casein to solidify or curdle.
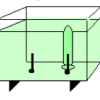
Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.

Biotech in a Bag
Source Institutions
In a series of three experiments, learners explore the basics of biotechnology using self-locking plastic baggies. Each experiment demonstrates a phenomenon or principle of biotechnology.

Fruity Electricity
Source Institutions
In this activity, Frankenstein's lab is running out of electricity! Learners use fruit to help Igor find a temporary source of energy to turn on a light.
