Search Results
Showing results 1 to 20 of 25

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Make a "Mummy"
Source Institutions
The Ancient Egyptians used a naturally-occurring salt from the banks of the Nile River, called natron, to mummify their dead.

We all Scream for Ice Cream
Source Institutions
In this activity, learners observe how salinity affects the freezing point of water by making and enjoying ice cream.
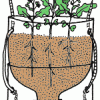
TerrAqua Investigation Column: What is the Land-Water Connection?
Source Institutions
In this investigation, learners plant seeds in a 2-liter bottle filled with soil that is connected to a water source below. Over the next few weeks, learners observe how the plants grow.

That Sinking Feeling
Source Institutions
In this quick activity, learners observe how salinity and temperature affect the density of water, to better understand the Great Ocean Conveyor.

Curious Crystals
Source Institutions
Learners carefully look at four known household crystals.

Crystal Creations: Grow Spikes of Crystals in the Sun
Source Institutions
This activity shows you how to make amazing crystal spikes using Epsom salt and the sun.
Let's Go Ice Fishing
Source Institutions
In this activity, learners are challenged to lift a floating ice cube out of a glass of water using just one end of a piece of string.

Crystal Stencil Stars
Source Institutions
In this activity on page 6 of the PDF, learners dissolve Epsom salt in water and discover that the resulting solution can be used to create a work of art.

Causes and Effects of Melting Ice
Source Institutions
In this activity, learners explore the concept of density-driven currents (thermohaline circulation) and how these currents are affected by climate change.

Crystals: Grow Your Own Garden
Source Institutions
In this simple activity (on page 2 of the PDF), learners make a crystal garden using salt, water, and a brick.

Salt Painting
Source Institutions
In this art meets chemistry activity, early learners discover the almost magical absorbent properties of salt while creating ethereal watercolor paintings.
Build A Hydrometer
Source Institutions
In this activity, learners will explore how a hydrometer works by building a working model and conducting experiments.

Crystal Painting
Source Institutions
In this activity, learners will "paint" their own crystal artwork by creating a picture with a super saturated salt solution.

Comparing Crystals
Source Institutions
In this chemistry activity (page 3 of the PDF), learners will learn about crystals by growing their very own.

Test Density with a Supersaturated Solution
Source Institutions
Learners create three solutions with different levels of salinity. They compare the density of these solutions by coloring them and layering them in a clear plastic cup and in a soda bottle.

Aluminum-Air Battery: Foiled again!
Source Institutions
Construct a simple battery that's able to power a small light or motor out of foil, salt water, and charcoal. A helpful video, produced by the Exploratorium, guides you along on this activity.

Water Wire: Electricity Flowing Through Water
Source Institutions
In this activity on page 10 of the PDF, learners detect the amount of energy that can flow through a sodium chloride electrolyte solution with a light sensor.
Salts & Solubility
Source Institutions
In this online interactive simulation, learners will add different salts to water and then watch the salts dissolve and achieve a dynamic equilibrium with solid precipitate.
