Search Results
Showing results 1 to 10 of 10

Why Circulate?
Source Institutions
In this activity related to the human circulatory system (on page 10 of the PDF), learners observe the dispersion of a drop of food coloring in water, draw conclusions about the movement of dissolved

Invisible Ink
Source Institutions
In this simple chemistry activity (page 1 of PDF under SciGirls Activity: Colorblind Dogs) about acids and bases, learners will mix a baking soda and water solution and use it to paint a message on a

We all Scream for Ice Cream
Source Institutions
In this activity, learners observe how salinity affects the freezing point of water by making and enjoying ice cream.

What's Your Blood Type?
Source Institutions
In this activity, learners perform a simulated blood test procedure.
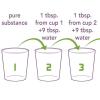
Sniffing for a Billionth
Source Institutions
This is an activity (located on page 4 of the PDF under What's Nano? Activity) about size and scale.

Glow Up
Source Institutions
In this activity, learners explore chemiluminescence and fluorescence. Learners examine 3 different solutions in regular light, in the dark with added bleach solution, and under a black light.

Make Your Own Soda Pop
Source Institutions
In this chemistry activity (page 8 of the PDF), learners will identify the instances of physical change, chemical change, and solutions while making homemade soda pop.

Making Naked Eggs: Eggs Without Shells
Source Institutions
This is an activity about acid-base reactions using eggs and vinegar. Learners place eggs inside a container of vinegar and leave to soak overnight.

Chemical Breath
Source Institutions
This is a chemistry lab activity about solutions (page 7 of the PDF). Learners see firsthand how chemicals in a solution can combine to form an entirely different substance.

See the Light
Source Institutions
Learners mix a solution of luminol with hydrogen peroxide to produce a reaction that gives off blue light.
