Search Results
Showing results 1 to 20 of 29

DNA Extraction
Source Institutions
In this activity related to plant biotechnology, learners extract DNA from fruit to investigate how it looks and feels.

Art with Salt and Ice
Source Institutions
This open-ended art project allows learners to create their own colorful ice sculpture by using rock salt and food coloring on a solid block of ice.

Kool Colors
Source Institutions
Learners investigate how temperature affects the rate of chemical reactions by observing how steel wool reacts with various types of Kool-Aid solutions at different temperatures.

Corals on Acid
Source Institutions
The objective of this inquiry-based lesson is for learners to gain an understanding of how increasing ocean acidity can affect the calcification of marine organisms.

Moisture Makers
Source Institutions
In this outdoor activity, learners compare the moisture released from different kinds of leaves and from different parts of the same leaf, by observing the color change of cobalt chloride paper.

Tabletop Biosphere
Source Institutions
In this activity, learners create a sealed, mini ecosystem that supplies freshwater shrimp with food, oxygen, and waste processing for at least three months.

Experimenting with Naked Eggs
Source Institutions
In this activity about osmosis, learners use a naked egg (one with a dissolved eggshell) to learn about selectively permeable membranes.

That Sinking Feeling
Source Institutions
In this quick activity, learners observe how salinity and temperature affect the density of water, to better understand the Great Ocean Conveyor.

How Boulders Are Born
Source Institutions
In this activity, learners review and discuss weathering, erosion and mass wasting, to gain a stronger understanding of how Hickory Run’s Boulder Field was formed after the Laurentide Continental Glac

Causes and Effects of Melting Ice
Source Institutions
In this activity, learners explore the concept of density-driven currents (thermohaline circulation) and how these currents are affected by climate change.
Ice Cube Painting
Source Institutions
In this activity, learners "draw" with frozen tempera paint. The ice cubes are prepared the day before by placing watered down tempera paint and popsicle sticks in ice cube trays.

Fossil Rocks
Source Institutions
In this activity, learners will discover how fossils are dug out of the earth by carefully exposing treasures hidden in an artificial rock that is easily made at home.
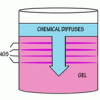
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

From the Ground Up
Source Institutions
In this plant science activity, learners conduct four experiments to observe how plants respond to sunlight and gravity.

Shimmering Lenses
Source Institutions
In this activity, learners use Jell-O to explore lenses. Learners cut Jell-O into convex and concave lens shapes and examine how light exits each lens in a darkened room.
Transparent Gelatin
Source Institutions
In this optics activity, learners explore how they can make gelatin stop light, but not stop them from seeing fruit suspended within.

Sinking Water
Source Institutions
In this experiment, learners float colored ice cubes in hot and cold water.

Nanoparticle Stained Glass
Source Institutions
In this activity/demo, learners are introduced to the connection between medieval stained glass artisans and nanotechnology.
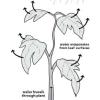
Plant Piping
Source Institutions
Learners build models to learn about the special cells and structures that plants use to move water from their roots up through the stems and leaves.

Diffusion & Osmosis with Data Analysis
Source Institutions
This three-part lab helps learners understand the essential principles governing diffusion and osmosis.
