Search Results
Showing results 1 to 10 of 10

Radioactive Decay of Candium
Source Institutions
In this simulation, learners use M&M™ candy to explore radioactive isotope decay.
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.

Rate of Solution Demonstration
Source Institutions
In this chemistry demonstration, learners investigate the factors that increase the rate of dissolution for a solid.

Ziptop Bag Chemistry
Source Institutions
In this chemistry activity, learners perform three chemical reactions in a sealed zip-top bag. Learners will record their observations and classify the changes as chemical or physical.

Ice Cream
Source Institutions
In this chemistry activity, learners use the lowered freezing point of water to chill another mixture (ice cream) to the solid state.

Combustion
Source Institutions
In this chemistry activity, learners discover that the weight of the product of combustion is greater than that of the starting material.
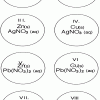
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

Double Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals to examine reactions that occur between two aqueous solutions.

What's in a Penny?
Source Institutions
In this chemistry activity, learners use chemical reactions to observe the composition of an alloy.

Cabbage Juice Indicator
Source Institutions
In this chemistry activity, learners make indicator solution from red cabbage. Then, learners test everyday foods and household substances using the cabbage juice indicator.
