Search Results
Showing results 141 to 160 of 318

Rocket Science
Source Institutions
Learners create a small explosion by collecting hydrogen and oxygen gas together and squeezing them into a flame.

"Boyle-ing" Water
Source Institutions
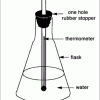
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Dip Dip, Hooray
Source Institutions
Lakes, streams and other freshwater bodies are a habitat for lots of living things, big and small.
Using Solar Energy
Source Institutions
In this activity, learners discover how solar energy can be used to heat water.

A Degrading Experience
Source Institutions
In this activity on page 27, learners perform an experiment to learn about how different types of marine debris degrade and how weather and sunlight affect the rate of degradation.

Crystal Gardens
Source Institutions
In this activity, which requires adult supervision, learners get to explore the awesome power of chemistry.

Water Quality and pH Levels in Aquatic Ecosystems
Source Institutions
In this fun and in depth hands-on experiment, learners test various liquid samples (distilled water, lemon juice, vinegar, and baking soda mixed with water) to determine their pH levels and identify e
Getting Windy
Source Institutions
In this activity, learners create and understand surface currents. Learners create example surface currents and discover how landmasses affect the current.

Window Under Water
Source Institutions
Glare from the sun and ripples from the wind can make it hard to see what's below the surface of a body of water.
Density Stackers
Source Institutions
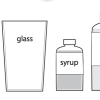
In this activity, learners investigate density as they discover how liquids separate to form density layers. Learners discover what happens when they add syrup, cooking oil, and water to a jar.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

Super Soaker
Source Institutions
In this activity (page 1 of the PDF under SciGirls Activity: Bogs), learners will test cups full of potting soil, sand, and sphagnum moss to see which earth material is able to soak up the most water.

Frosty Glasses
Source Institutions
In this activity, learners explore why frost forms. They create their own frost using a solution of ice water and salt in a glass.

Automotive Emissions and the Greenhouse Effect
Source Institutions
In this activity about global climate change, learners will conduct an experiment and collect data to compare the amount of carbon dioxide (CO2) in four different sources of gases.
Weathering and Erosion
Source Institutions
In this multi-station lab, learners conduct a series of experiments to explore the processes and effects of weathering and erosion.

Corals and Chemistry
Source Institutions
In this activity, learners investigate how increased carbon dioxide (CO2) emissions from the burning of fossil fuels is changing the acidity (pH) of the ocean and affecting coral reefs and other marin

What is a “Convection Cell”?
Source Institutions
In this demonstration, learners can observe a number of small convection cells generated from a mixture of aluminum powder and silicon oil on a hot plate.
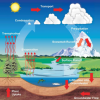
Weather Stations: Phase Change
Source Institutions
In this activity, learners observe the water cycle in action! Water vapor in a tumbler condenses on chilled aluminum foil — producing the liquid form of water familiar to us as rain and dew.

Let's Dew It!
Source Institutions
From the Weather Watchers featured theme on the CYBERCHASE website. Learners will conduct experiments to discover how air temperature and humidity work together to make condensation, dew, and fog.

Trash Traits
Source Institutions
In this activity on page 24, learners perform experiments to examine whether or not trash can float, blow around, or wash away.
