Search Results
Showing results 101 to 120 of 156
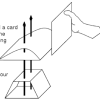
Wingin' It
Source Institutions
Learners explore the Bernoulli effect by building an airfoil (airplane wing) and making it fly.

Hanford at the Half-Life Radiation Calculator
Source Institutions
This quiz lets you estimate your annual radiation exposure.

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.

Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

Save Your Ears
Source Institutions
This game depicts a woman going through her day, faced with various loud sounds.

Iron in Cereal: Find iron in your food!
Source Institutions
Learners investigate an iron-fortified cereal by stirring it with a strong magnet. They discover that metallic iron is present in some cereals.
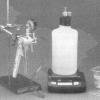
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.

What's That Sound?
Source Institutions
This game plays a dozen different sounds, altered to simulate what they would sound like if you had hearing loss.
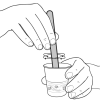
Choose Your Ooze
Source Institutions
During this activity, learners will make different versions of "ooze" using varied proportions of detergent and glue.

History of Electricity
Source Institutions
This is a series of demonstrations about different electrical and magnetic phenomena.

Miscibility
Source Institutions
Learners observe a bottle containing water and oil. They are invited to pick up the bottle and mix the contents together.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Pollution Diffusion
Source Institutions
Learners design their own experiment to investigate how pollution diffuses through ground material.

Currently Working
Source Institutions
Learners test solutions of water, sugar, salt, and hydrochloric acid for electrical conductivity. They immerse leads from a lighting device (a battery pack connected to an LED) into each solution.
Take Out the Trash
Source Institutions
Learners explore how recyclers take advantage of the different properties of materials, such as magnetism and density, to separate them from a mixture.
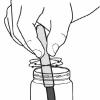
Salting Out
Source Institutions
In this activity, learners create a mixture of water, alcohol and permanent marker ink, and then add salt to form a colored alcohol layer on top of a colorless water layer.

Dye Detective
Source Institutions
Learners use filter paper and water to analyze six different markers. They mark the paper with ink, and dip the paper in water. The water travels up the paper and dissolved ink travels with it.

Spicy Indicator: Use turmeric to test for bases in your home
Source Institutions
This activity uses turmeric, a common spice in curry, as an indicator for acidity and basicity. Turmeric is yellow in acid and neutral substances, but turns bright red with bases.

Pot-in-Pot Refrigeration
Source Institutions
In this activity (on page 2 of PDF), learners create a low-tech refrigerator that requires no electricity to keep food from spoiling.

Diving Submarine
Source Institutions
Learners use a commercially available toy to experiment with density. They fill a chamber in the toy submarine with baking powder and release it into a tank of water.
