Search Results
Showing results 1 to 20 of 45

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

Collect Oxygen Over Water
Source Institutions
In this activity, learners use a pneumatic trough (see related activity) to generate and collect pure oxygen.
Trading Places: Redox Reactions
Source Institutions
Visitors add drops of copper sulfate solution onto a steel nail. They observe the nail change color from silver to brown as the copper plates onto the nail.
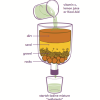
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Kool Colors
Source Institutions
Learners investigate how temperature affects the rate of chemical reactions by observing how steel wool reacts with various types of Kool-Aid solutions at different temperatures.

Making a Battery from a Potato
Source Institutions
In this electrochemistry activity, young learners and adult helpers create a battery from a potato to run a clock.

Disappearing Colors
Source Institutions
In this challenge, learners figure out how to make a juice stain disappear.

Fast Rusting
Source Institutions
In this activity, learners conduct an experiment to find out if steel wool will weigh more or less when it is burned. Learners will explore the effects of oxidation and rusting on the steel wool.
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.

Fuel for Living Things
Source Institutions
In this activity, learners observe what happens when yeast cells are provided with a source of food (sugar). Red cabbage "juice" will serve as an indicator for the presence of carbon dioxide.

The Carbon Cycle: How It Works
Source Institutions
In this game, learners walk through an imaginary Carbon Cycle and explore the ways in which carbon is stored in reservoirs and the processes that transport the carbon atom from one location to another

Silver Crystals
Source Institutions
This is written as a static display, but can easily become a hands-on experiment for learners.

Trading Places
Source Institutions
In this activity, learners discover that atoms and ions of different metals will change places.

Lifting Lemon
Source Institutions
In this physics demonstration, learners will be surprised when a lemon slice appears to magically levitate within a pint glass.

Green Pennies
Source Institutions
In this activity, learners create their own experiment and test which of 4 mixtures of household chemicals turn pennies green over 5 days.
Penny for Your Thoughts
Source Institutions
In this activity, learners will explore how metals react with each other. They will see these metals change before their eyes as they coat a paperclip with the copper taken from a penny.

Copper Caper
Source Institutions
In this activity, learners conduct an oxidation experiment that turns old pennies bright and shiny. Learners soak 20 dull, dirty pennies in a bowl of salt and vinegar for five minutes.

Treasures in the Rough
Source Institutions
In this archaeology activity, learners make observations and conduct an experiment to demonstrate the effect saltwater has on artifacts.

Gravestone Weathering
Source Institutions
In this activity (located on pages 9-14 of PDF), learners visit a cemetery to examine the distinguishing characteristics of rock weathering.

Changing Colors
Source Institutions
In this challenge, learners have to figure out in what order to combine five solutions to change the color from clear, to yellow, to blue, and back to clear.
