Search Results
Showing results 381 to 400 of 799

Glow Up
Source Institutions
In this activity, learners explore chemiluminescence and fluorescence. Learners examine 3 different solutions in regular light, in the dark with added bleach solution, and under a black light.

Build a Rocket - and a Launch Pad!
Source Institutions
In this activity, learners construct a rocket powered by the pressure generated from an effervescing antacid tablet reacting with water, and build a launch pad for their rocket.

Air, It's Really There
Source Institutions
This lesson focuses on molecular motion in gases. Learners compare the mass of a basketball when it is deflated and after it has been inflated.

Stuck on You: Adhesion
Source Institutions
Learners explore water adhesion and learn about why water molecules are more strongly attracted to some substances than others.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.
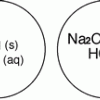
Gas Producing Micro-Reaction
Source Institutions
In this chemistry activity, learners use common chemicals and metals to examine reactions that produce gaseous substances.

Stained Glass Glue
Source Institutions
In this activity on page 6 of the PDF, learners use glue instead of glass to create artwork that can be hung in a window.

Iodine Investigators!
Source Institutions
In this activity on page 7 of the PDF (Chemistry—It’s Elemental), learners use iodine to identify foods that contain starch.

Exploring A Hydrogel
Source Institutions
In this activity on page 10 of the PDF, learners develop an experiment to answer the following question: "How much water can the hydrogel in a baby diaper hold?" Use this activity to explore polymers,

Sticky Structures
Source Institutions
In this engineering/design/arts and crafts activity, learners design and build "platforms" or "bridges" that can hold weight, and test which glue makes the strongest structure.

Your Energy Needs
Source Institutions
In this activity about the relationship between food and energy (page 8 of PDF), learners estimate average daily baseline energy (Calorie) needs and energy needs for different levels of activity.
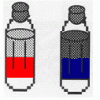
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

Try Your Hand at Nano
Source Institutions
This lesson focuses on two simple activities that younger learners can do to gain an appreciation of nanotechnology. First, learners measure their hands in nanometers.
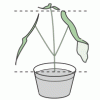
Do Plants Need Light?
Source Institutions
In this food science activity, learners conduct an experiment that demonstrates the importance of light to plants.

Dancing Spaghetti
Source Institutions
In this chemistry activity, learners use spaghetti to explore density and chemical reactions.

Production of Oxygen
Source Institutions
In this chemistry activity, learners use yeast and hydrogen peroxide to generate a gas (oxygen) and test some of its properties.

Shrinkers
Source Institutions
In this hands-on activity, learners use heat to shrink samples of polystyrene plastic (#6 recycle code). Learners compare the size and shape of the plastic pieces before and after shrinking.

Concentrate!
Source Institutions
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution.

Make Your Own Batteries!
Source Institutions
This activity (on page 3 of the PDF under GPS: Body Electricity Activity) is a full inquiry investigation into conductivity.

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.
