Search Results
Showing results 201 to 220 of 292

Smell the Difference
Source Institutions
In this two-part activity, learners use household items to smell the difference between some stereoisomers, or molecules which are mirror images of one another.

Ice Cream
Source Institutions
In this chemistry activity, learners use the lowered freezing point of water to chill another mixture (ice cream) to the solid state.

Powder Particulars
Source Institutions
In this introductory activity and demonstration, learners are introduced to the concept that different substances react chemically in characteristic ways.

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.

Testing Vitamin C: Chemistry's Clear Solution
Source Institutions
In this activity on page 8 of the PDF, learners investigate vitamin C. Learners conduct a chemistry experiment to determine if Tang drink mix or orange juice contains more vitamin C.

Temperature vs. Height: Soda Geyser Series #6
Source Institutions
In this activity, learners conduct a controlled experiment to examine how temperature will affect the height of a soda geyser.
Bird Feeder
Source Institutions
In this outdoor activity, learners construct bird feeders and set them up at to investigate bird behavior for one or two weeks. Multiple feeder designs are suggested.

Reflective Solar Cooker
Source Institutions
In this activity, learners use the Sun's energy to cook marshmallows. Learners construct the solar oven out of simple everyday materials.
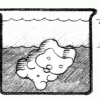
Gummy Growth
Source Institutions
In this activity related to Archimedes' Principle, learners use water displacement to compare the volume of an expanded gummy bear with a gummy bear in its original condition.

Comparing the Density of Different Liquids
Source Institutions
Learners carefully pour vegetable oil, water, and corn syrup in any order into a cup and discover that regardless of the order they are poured, the liquids arrange themselves in layers the same way.

Do the Mystery Samples Contain Life?
Source Institutions
In this activity (on pages 13-16 of the PDF) learners investigate three mystery samples to see which one contains life. The three samples are sand, sand and yeast, and sand and antacid.

Fruity Electricity
Source Institutions
In this activity, Frankenstein's lab is running out of electricity! Learners use fruit to help Igor find a temporary source of energy to turn on a light.

Ship the Chip
Source Institutions
In this activity, learners explore engineering package designs that meet the needs of safely shipping a product.

Thymus DNA Extractions
Source Institutions
This laboratory exercise is designed to show learners how DNA can be extracted from a chunk of thymus (sweetbread) or liver.

Soda Brand vs. Height Experiment: Soda Geyser Series #4
Source Institutions
In this activity, learners conduct a controlled experiment to examine which brand of soda makes the best (highest) soda geyser.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Gummy Shapes
Source Institutions
In this activity, learners use chemistry to “self-assemble” gummy shapes. Learners discover that self-assembly is a process by which molecules and cells form themselves into functional structures.

Radioactive Decay of Candium
Source Institutions
In this simulation, learners use M&M™ candy to explore radioactive isotope decay.

Erupting Fizz
Source Institutions
This is a highly visual demonstration that illustrates both the effects of density and chemical reactions.

Cookie Surface Area
Source Institutions
This is an activity (on page 2 of the PDF under Surface Area Activity) about surface area to volume ratio.
