Search Results
Showing results 141 to 160 of 372
The Nose Knows!
Source Institutions
In this activity on page 9 of the PDF, learners test how flavoring extracts move through the walls of a balloon.

Moo Glue
Source Institutions
Using a milk-based recipe, learners create "moo glue" which is basically white school-type glue. The "secret ingredient" in milk that helps make glue is a chemical called casein.

Leaning Tower of Pasta
Learners build structures from spaghetti and marshmallows to determine which structures are able to handle the greatest load.
Why is the Sky Blue?
Source Institutions
In this activity, learners create a "mini sky" in a glass of water in a dark room.

Veggies with Vigor
Source Institutions
In this activity, learners try to revive wilted celery. Learners discover that plants wilt when their cells lose water through evaporation. Use this activity to introduce capillary action.

Glowing Pickle
Source Institutions
In this activity, high voltage is applied across a pickle to emit a yellow glow. This activity should only be conducted by skilled adults and is best suited as a demonstration.

Hot & Cold
Source Institutions
In this activity, learners experiment with hydrogen peroxide, vinegar, yeast, and baking soda to produce hot and cold reactions. Use this activity to demonstrate exothermic and endothermic reactions.

Space Jell-O
Source Institutions
Albert Einstein proved that space bends around anything that has mass. This activity uses Jell-O's ability to bend around objects as a model for space bending around planets and stars.
Transparent Gelatin
Source Institutions
In this optics activity, learners explore how they can make gelatin stop light, but not stop them from seeing fruit suspended within.

Cauldron Bubbles
Source Institutions
In this activity, learners mix up a bubbly brew and examine density. Learners explore how they can make different materials fall and rise in water using oil, water, and salt.

Diffusion of Water with Gummy Bears
Source Institutions
In this activity, learners investigate the movement of water into and out of a polymer. Learners test the diffusion of water through gummy bears, which are made of sugar and gelatin (a polymer).
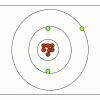
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).

Edible Ink
Source Institutions
In this chemistry activity (page 6 of the PDF), learners observe a chemical change. Learners write and reveal a secret message using edible ink.

Bready Bubble Balloon
Source Institutions
Learners discover the bubble power of living cells in this multi-hour experiment with baker's yeast. Learners make a living yeast/water solution in a bottle, and add table sugar to feed the yeast.

Nano Ice Cream
Source Institutions
In this activity/demo, learners discover how liquid nitrogen cools a creamy mixture at such a rapid rate that it precipitates super fine grained (nano) ice cream.

Mystery Powders
Source Institutions
In this activity on page 2 of the PDF (Get Cooking With Chemistry), learners conduct chemical tests on certain powders used in cooking.

Neutralizing Acids and Bases
Source Institutions
Learners use their knowledge of color changes with red cabbage indicator to neutralize an acidic solution with a base and then neutralize a basic solution with an acid.

Lighting Up Celery Stalks
Source Institutions
In this activity, learners conduct a series of hands-on experiments that demonstrate how the working of plants' veins, known as capillary action, enables water to travel throughout the length of a pla

Dancing Cereal
Source Institutions
In this quick activity (on page 2 of the PDF under GPS: Body Electricity Activity), learners will observe how dry breakfast cereal appears to dance when it gets close to a balloon charged with static

Ice Cream Freeze
Source Institutions
In this fun and delicious chemistry activity (page 1 of the PDF), learners will explore the difference between physical and chemical change by making homemade ice cream.
