Search Results
Showing results 1 to 20 of 35

Burn a Peanut
Source Institutions
In this activity, learners burn a peanut, which produces a flame that can be used to boil away water and count the calories contained in the peanut.

Iron for Breakfast
Source Institutions
Did you know that some breakfast cereals are fortified with ferric phosphate, while others contain tiny pieces of reduced iron?

Homemade Butter
Source Institutions
In this activity, learners will turn cream and salt into butter—using marbles. Learners will explore how shaking up fat globules help them create homemade butter.

Iodine Investigators!
Source Institutions
In this activity on page 7 of the PDF (Chemistry—It’s Elemental), learners use iodine to identify foods that contain starch.

Spit Test
Source Institutions
In this biology activity (page 8 of the PDF), learners will explore how saliva assists in the beginning of the digestive process.

A Feast for Yeast
Source Institutions
In this activity on page 6 of the PDF (Get Cooking With Chemistry), learners investigate yeast. Learners prepare an experiment to observe what yeast cells like to eat.

Soap Bubble Art
Source Institutions
Capture soap bubble patterns on paper! In this activity, learners can create beautiful pictures from popping soap bubbles.

Plant Power
Source Institutions
In this chemistry challenge, learners identify which plants have the enzyme "catalase" that breaks hydrogen peroxide into water and oxygen.

Pickle-oh!: Musical Pickle Instrument
Source Institutions
What's a Pickle-Oh? Two pieces of pickle on a stick are connected to a Pico Cricket (micro controller). When you slide the pickles apart the note changes.

Sweet Measurements
Source Institutions
In this activity on page 3 of the PDF, learners investigate how much sugar is in a soda. Learners use sugar cubes to measure and calculate the amount of sugar in a bottle of soda.

Cabbage Patch Chemistry
Source Institutions
In this chemistry activity, learners will learn how to make their own pH indicator using cabbage leaves, and then test common household items with their homemade indicator.

Experimenting with Naked Eggs
Source Institutions
In this activity about osmosis, learners use a naked egg (one with a dissolved eggshell) to learn about selectively permeable membranes.

Make a Green Gumball Black
Source Institutions
In this optics activity, learners use a shoebox, colored cellophane and sunlight to "change" the colors of gumballs. Learners will be surprised when the green and blue gumballs appear black!
The Nose Knows!
Source Institutions
In this activity on page 9 of the PDF, learners test how flavoring extracts move through the walls of a balloon.

Dancing Cereal
Source Institutions
In this quick activity (on page 2 of the PDF under GPS: Body Electricity Activity), learners will observe how dry breakfast cereal appears to dance when it gets close to a balloon charged with static

Freezing Lakes
Source Institutions
In some parts of the world, lakes freeze during winter. In this activity learners will explore water’s unique properties of freezing and melting, and how these relate to density and temperature.

Making Naked Eggs: Eggs Without Shells
Source Institutions
This is an activity about acid-base reactions using eggs and vinegar. Learners place eggs inside a container of vinegar and leave to soak overnight.
Chew that Gum
Source Institutions
In this quick activity (page 1 of PDF under SciGirls Activity: Exercise and Memory), learners will investigate what happens to bubble gum when it is chewed for 5-10 minutes.

A Recipe for Air
Learners use M&Ms® (or any other multi-color, equally-sized small candy or pieces) to create a pie graph that expresses the composition of air.
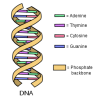
Close, Closer, Closest
Source Institutions
In this activity, learners perform an experiment that models a chromatography-like process called electrophoresis, a process used to analyze DNA.
