Search Results
Showing results 121 to 140 of 176
Mystery Writing: Write and develop a secret message
Source Institutions
Learners write an invisible message using lemon juice on a piece of paper. They then develop the message by soaking the paper in a dilute iodine solution.

Racing M&M Colors
Source Institutions
Learners design their own experiment to determine which M&M color dissolves the fastest in water.
Build a Super Structure
Source Institutions
In this activity, learners use things from the kitchen as building materials to explore how shapes contribute to the strength of different structures.

Smell the Difference
Source Institutions
In this two-part activity, learners use household items to smell the difference between some stereoisomers, or molecules which are mirror images of one another.

Ice Cream
Source Institutions
In this chemistry activity, learners use the lowered freezing point of water to chill another mixture (ice cream) to the solid state.

Powder Particulars
Source Institutions
In this introductory activity and demonstration, learners are introduced to the concept that different substances react chemically in characteristic ways.

Kimchee Fermentation Chamber
Source Institutions
Learners make kimchee or sauerkraut, which is really just fermented cabbage, in a 2-liter plastic bottle.

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.

Ice Cream Shake
Source Institutions
In this tasty activity, learners make their own ice cream any day of the year in an exploration of heat and cold. Highlights include freezing and melting and the transition from liquid to solid.

Reflective Solar Cooker
Source Institutions
In this activity, learners use the Sun's energy to cook marshmallows. Learners construct the solar oven out of simple everyday materials.
Gummy Growth
Source Institutions
In this activity related to Archimedes' Principle, learners use water displacement to compare the volume of an expanded gummy bear with a gummy bear in its original condition.

Spicy Indicator: Use turmeric to test for bases in your home
Source Institutions
This activity uses turmeric, a common spice in curry, as an indicator for acidity and basicity. Turmeric is yellow in acid and neutral substances, but turns bright red with bases.

Comparing the Density of Different Liquids
Source Institutions
Learners carefully pour vegetable oil, water, and corn syrup in any order into a cup and discover that regardless of the order they are poured, the liquids arrange themselves in layers the same way.

Fruity Electricity
Source Institutions
In this activity, Frankenstein's lab is running out of electricity! Learners use fruit to help Igor find a temporary source of energy to turn on a light.
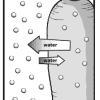
Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.

Say Cheese!
Source Institutions
Create a chemical reaction that makes cheese! This hands-on activity demonstrates that molecules and atoms are tiny particles that make up everything around us.

Radioactive Decay of Candium
Source Institutions
In this simulation, learners use M&M™ candy to explore radioactive isotope decay.

Erupting Fizz
Source Institutions
This is a highly visual demonstration that illustrates both the effects of density and chemical reactions.

Testing Falling Peanut Butter Sandwich Myth
Source Institutions
In this activity related to rotational inertia (page 1 of the PDF under SciGirls Activity: Microgravity), learners will use a bit of scientific experimenting to test if open-faced peanut butter sandwi

Copper Cleanup
Source Institutions
In this hands-on experiment, kids use chemistry to explore whether acids or bases are better at restoring a penny’s shine.
