Search Results
Showing results 121 to 140 of 306

Ice Cream Freeze
Source Institutions
In this fun and delicious chemistry activity (page 1 of the PDF), learners will explore the difference between physical and chemical change by making homemade ice cream.

Mummy Magic
Source Institutions
Make your own mummy! Using a combination of salts, transform an apple into a mummy and discover how the Ancient Egyptians used drying as one step in the mummification process.

Pepper Scatter
Source Institutions
In this quick activity, learners break the tension that happens when water develops a "skin." Learners use water, pepper and some soap to discover the wonders of surface tension—the force that attract

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.

Get the Porridge Just Right
Source Institutions
Learners set up three different bowls, each with a different mass of oatmeal. Learners monitor the temperature of the oatmeal and find that larger masses take longer to cool.

Find the Fizz: Discover the Secret of Baking Powder
Source Institutions
In this activity on page 4 of the PDF (Get Cooking With Chemistry), learners investigate ingredients that combine to produce gas bubbles.

Shark Sense of Smell
Source Institutions
This is an activity about our sense of smell and how it compares to sharks' super noses. Learners will create varying solutions of water and perfume.

Colors Collide or Combine
Source Institutions
Learners place multiple M&M's in a plate of water to watch what happens as the candies dissolve.

Cooking with Chemistry
Source Institutions
In this activity, learners experiment with different variables in making hollandaise sauce to achieve the correct texture and consistency.

Mold Growth
Source Institutions
In this activity learners observe mold growth on different types of bread by measuring and recording the growth rate.

Mysterious M&M's
Source Institutions
Learners place an M&M candy in water and observe what happens. The sugar-and-color coating dissolves and spreads out in a circular pattern around the M&M.

Color Changes with Acids and Bases
Source Institutions
Learners mix a variety of substances with red cabbage juice. The juice changes color to indicate whether each substance is an acid or a base.
Air-filled (Pneumatic) Bone Experiments
Source Institutions
Just like birds, some dinosaurs had air-filled (pneumatic) bones, which made the dinosaurs' skeletons lighter.

Wheat Evolution: Dough Washing
Source Institutions
In this activity (Page 22 of PDF), learners investigate the evolution of wheat by washing different types of dough with water and comparing the results.

Investigating the Line
Source Institutions
In the related activity called "Colors Collide or Combine," learners are intrigued by the apparent "line" that forms where colors from M&M coatings meet but do not mix.

Fizzy Fun
Source Institutions
In this activity, learners test what happens when they put baking power on different frozen liquids.
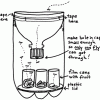
Build a Fruit Fly Trap
Source Institutions
In this construction activity, students use a 2-liter bottle to build a fly trap.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.

Dancing Spaghetti
Source Institutions
In this chemistry activity, learners use spaghetti to explore density and chemical reactions.

Make Your Own Soda Pop
Source Institutions
In this chemistry activity (page 8 of the PDF), learners will identify the instances of physical change, chemical change, and solutions while making homemade soda pop.
