Search Results
Showing results 1 to 17 of 17

Candy Chemosynthesis
Source Institutions
In this activity, groups of learners work together to create edible models of chemicals involved in autotrophic nutrition.

Hollandaise Sauce: Emulsion at Work
Source Institutions
In this activity, learners follow a recipe to make hollandaise sauce. Learners discover how cooks use egg yolks to blend oil and water together into a smooth mix.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!
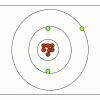
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).

Sweetly Balanced Equations
Source Institutions
In this (edible) activity, learners balance chemical equations using different kinds and colors of candy that represent different atoms. Learners will work in pairs and explore conservation of atoms.

Mighty Molecules
Source Institutions
In this activity, learners use marshmallows and gum drops to construct seven models of molecules. Learners classify (solid, liquid or gas) and draw diagrams of the molecules.

Jiggly Jupiter
Source Institutions
In this activity, learners build edible models of Jupiter and Earth to compare their sizes and illustrate the planets' internal layers.

Dye Like A Natural
Source Institutions
In this activity, learners stain fabrics--on purpose!

Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

Column Chromatography
Source Institutions
In this activity, learners separate the components of Gatorade using a home-made affinity column.

Nuclear Fusion
Source Institutions
This simple and engaging astronomy activity explains nuclear fusion and how radiation is generated by stars, using marshmallows as a model.

Smell the Difference
Source Institutions
In this two-part activity, learners use household items to smell the difference between some stereoisomers, or molecules which are mirror images of one another.

Molecular Menagerie
Source Institutions
In this activity, learners use molecular model kits to construct familiar molecules like lactose, caffeine, and Aspirin.

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.
Finding the Carbon in Sugar
Source Institutions
In this activity about combustion and energy, learners observe a burning candle in a sealed jar and the burning of white sugar.

Atoms and Matter (K-2)
Source Institutions
In this activity, learners explore atoms as the smallest building blocks of matter. With adult help, learners start by dividing play dough in half, over and over again.
Properties of Metals
Source Institutions
In this activity, learners explore the properties of metals at four stations. The stations include A) Magnetism and Breakfast Cereal; B) Conductivity of Metals; C) Alloys; and D) Metal Plating.
