Search Results
Showing results 1 to 20 of 35

Candy Chemosynthesis
Source Institutions
In this activity, groups of learners work together to create edible models of chemicals involved in autotrophic nutrition.

Plankton Feeding
Source Institutions
This activity provides a hands-on experience with a scale model, a relatively high viscosity fluid, and feeding behaviors.

Dunking the Planets
Source Institutions
In this demonstration, learners compare the relative sizes and masses of scale models of the planets as represented by fruits and other foods.
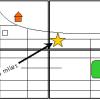
Solar System in My Neighborhood
Source Institutions
In this activity, learners shrink the scale of the vast solar system to the size of their neighborhood.

Bury Me Not!
Source Institutions
This activity (page 2 of the PDF under SciGirls Activity: Bogs) is a full inquiry investigation into decomposition.

Pie-Pan Convection
Source Institutions
It's difficult to see convection currents in any liquid that's undergoing a temperature change, but in this Exploratorium Science Snack, you can see the currents with the help of food coloring.
Pepper Scatter
Source Institutions
In this activity, learners explore the forces at work in water. Learners experiment to find out what happens to pepper in water when they touch it with bar soap and liquid detergent.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!
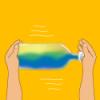
Making Waves
Source Institutions
Investigate the interaction of liquids of different densities and experiment with wave patterns with this hands-on activity.

Space Jell-O
Source Institutions
Albert Einstein proved that space bends around anything that has mass. This activity uses Jell-O's ability to bend around objects as a model for space bending around planets and stars.

Pepper Scatter
Source Institutions
In this quick activity, learners break the tension that happens when water develops a "skin." Learners use water, pepper and some soap to discover the wonders of surface tension—the force that attract

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.

Lifting Lemon
Source Institutions
In this physics demonstration, learners will be surprised when a lemon slice appears to magically levitate within a pint glass.

Why is the Sky Blue?
Source Institutions
In this activity, learners use a flashlight, a glass of water, and some milk to examine why the sky is blue and sunsets are red.

Jiggly Jupiter
Source Institutions
In this activity, learners build edible models of Jupiter and Earth to compare their sizes and illustrate the planets' internal layers.
How Does Water Climb a Tree?
Source Institutions
In this activity, learners conduct an experiment to explore how water flows up from a tree's roots to its leafy crown.

Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

What's So Special about Water: Solubility and Density
Source Institutions
In this activity about water solubility and density, learners use critical thinking skills to determine why water can dissolve some things and not others.

Capturing Carbon Dioxide
Source Institutions
In this activity, learners investigate carbon sequestration by creating a carbonated beverage out of apple juice and dry ice.

Nuclear Fusion
Source Institutions
This simple and engaging astronomy activity explains nuclear fusion and how radiation is generated by stars, using marshmallows as a model.
