Search Results
Showing results 1 to 15 of 15

pH Scale
Source Institutions
In this online interactive simulation, learners will test the pH of liquids like coffee, spit, and soap to determine whether each is acidic, basic, or neutral.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).
Sniffing for a Billionth
Source Institutions
This is an activity (located on page 4 of the PDF under What's Nano? Activity) about size and scale.

The Colors of Flowers
Source Institutions
In this activity, learners perform an experiment to find out what determines a flower's color.

Nature of Dye
Source Institutions
"Nature of Dye" allows participants to create their own dyes and art while exploring how chemicals interact and how these interactions can have real-world applications.

Hot Stuff!: Testing for Carbon Dioxide from Our Own Breath
Learners blow into balloons and collect their breath--carbon dioxide gas (CO2). They then blow the CO2 from the balloon into a solution of acid-base indicator.

Moving Without Wheels
In a class demonstration, learners observe a simple water cycle model to better understand its role in pollutant transport.

Exploring Size: Scented Solutions
Source Institutions
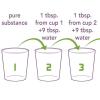
This is an activity in which learners will find that they can detect differences in concentration better with their nose (smelling) than with their eyes (seeing).

Acid Rain Effects
Learners conduct a simple experiment to model and explore the harmful effects of acid rain (vinegar) on living (green leaf and eggshell) and non-living (paper clip) objects.

Hot Stuff!: Creating and Testing for Carbon Dioxide
In this demonstration, learners observe vinegar and baking soda reacting to form carbon dioxide (CO2) gas.

Hot Stuff!: Testing Ice
In this demonstration, learners compare and contrast regular water ice to dry ice (frozen carbon dioxide). Both samples are placed in a solution of acid-base indicator.

Sugar Crystal Challenge
Source Institutions
This lesson focuses on surface area and how the shape of sugar crystals may differ as they are grown from sugars of different coarseness.

Avogadro's Bubbly Adventure
Source Institutions
In this activity on page 7 of the PDF, learners investigate the solubility of gas in water at different temperatures. This experiment will help learners determine if temperature affects solubility.

Water Treatment
Source Institutions
Water treatment on a large scale enables the supply of clean drinking water to communities.
