Search Results
Showing results 1 to 20 of 23

Exploring Size: Scented Solutions
Source Institutions
This is an activity in which learners will find that they can detect differences in concentration better with their nose (smelling) than with their eyes (seeing).

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.

Shrinking Cups
Source Institutions
This is a quick activity (on page 2 of the PDF under Gecko Feet Activity) about the forces of gravity and surface tension and how their behavior is influenced by size.
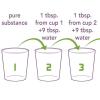
Sniffing for a Billionth
Source Institutions
This is an activity (located on page 4 of the PDF under What's Nano? Activity) about size and scale.

Gravity Fail
Source Institutions
In this activity, learners try pouring water out of a regular cup and a miniature cup. It’s harder than it sounds! Learners discover that different forces dominate at different size scales.

Ready, Set, Fizz!
Source Institutions
In this activity, learners explore the chemical reaction between water and effervescent antacid tablets. This hands-on activity models how a material can act differently when it's nanometer-sized.

Exploring Properties: Surface Area
Source Institutions
This hands-on activity demonstrates how a material can act differently when it's nanometer-sized.

Exploring Products: Nano Fabrics
Source Institutions
In this activity, learners explore how the application of nano-sized "whiskers" can protect clothing from stains.

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).

Sand, Plants and Pants
Source Institutions
In this activity, learners explore how the application of nano-sized particles or coatings can change a bigger material’s properties.

Shrinkers
Source Institutions
In this hands-on activity, learners use heat to shrink samples of polystyrene plastic (#6 recycle code). Learners compare the size and shape of the plastic pieces before and after shrinking.

Shrinkers: Cook up some plastic!
Source Institutions
In this activity (on page 2 of the PDF), learners (with adult help and supervision) investigate how heat affects polystyrene plastic.
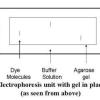
Gel Electrophoresis of Dyes
Source Institutions
In this experiment related to plant biotechnology, learners discover how to prepare and load an electrophoresis gel.

Exploring the Nanoworld with LEGO Bricks: Structure-Property Relationships at the Nanoscale
Source Institutions
In this activity (pages 32-41), learners learn how the atomic and molecular arrangement of matter are related to physical properties.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Surface Area and Soda Geysers
Source Institutions
This is an activity (located on page 4 of the PDF under Surface Area Activity) about surface area and reactivity.

That's the Way the Ball Bounces: Level 1
Source Institutions
In this activity, learners prepare four polymer elastomers and then compare their physical properties, such as texture, color, size, and bounce height.

How Big is Small
Source Institutions
In this classic hands-on activity, learners estimate the length of a molecule by floating a fatty acid (oleic acid) on water.

Sugar Crystal Challenge
Source Institutions
This lesson focuses on surface area and how the shape of sugar crystals may differ as they are grown from sugars of different coarseness.
