Search Results
Showing results 41 to 60 of 110
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Exploring Products: Nano Fabrics
Source Institutions
In this activity, learners explore how the application of nano-sized "whiskers" can protect clothing from stains.
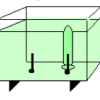
Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.
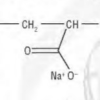
Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.

Forgotten Genius
Source Institutions
This series of chemistry stations is designed to accompany the PBS documentary about African-American chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or p
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).
Foam Peanuts
Source Institutions
Learners compare the properties and solubilities of Styrofoam (TM), ecofoam packing peanuts, and popcorn. First, the solubility of each substance is tested in water.
Egg Osmosis
Source Institutions
Visitors observe three beakers. One beaker contains an egg immersed in vinegar. Visitors observe carbon dioxide gas escaping from the shell as the calcium carbonate reacts with the vinegar.

Lotus Leaf Effect
Source Institutions
This is a demonstration about how nature inspires nanotechnology. It is easily adapted into a hands-on activity for an individual or groups.

Dip Dip, Hooray
Source Institutions
Lakes, streams and other freshwater bodies are a habitat for lots of living things, big and small.

Make a Lake
Source Institutions
Where rainwater goes after the rain stops? And why there are rivers and lakes in some parts of the land but not in others?
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

A Crayon Rock Cycle- Metamorphic
Source Institutions
This is part 2 of the three-part "Crayon Rock Cycle" activity and must be done after part 1: Sedimentary Rocks. In this activity, learners explore how metamorphic rocks form.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.

Burning Issues
Source Institutions
Learners use a candle to investigate the products of combustion. When a glass rod is held over a lit candle, the candle flame deposits carbon on the rod.

The Watershed Connection
Source Institutions
In this activity, learners interact with a 3-D model of a watershed to better understand the interconnectedness of terrestrial and aquatic environments.

Chemical Footprint—Family Activity
Source Institutions
In this multi-part activity learners examine non-point water pollution.
