Search Results
Showing results 1 to 20 of 26

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.

Changing the Density of a Liquid: Heating and Cooling
Source Institutions
Learners investigate how the temperature of water affects its density.

Changing the Density of a Liquid: Adding Salt
Source Institutions
Learners see that a carrot slice sinks in fresh water and floats in saltwater.

Dissolving Different Liquids in Water
Source Institutions
In this activity, learners add different liquids to water and apply their working definition of “dissolving” to their observations.

From Gas to Liquid to Solid
Source Institutions
What causes frost to form on the outside of a cold container? In this activity, learners discover that liquid water can change states and freeze to become ice.

Colors Collide or Combine
Source Institutions
Learners place multiple M&M's in a plate of water to watch what happens as the candies dissolve.

Exploring Moisture on the Outside of a Cold Cup
Source Institutions
In this activity, learners explore the relationship between cooling water vapor and condensation. Learners investigate condensation forming on the outside of a cold cup.

Does Size Make a Difference?
Source Institutions
In this activity on page 15 of the PDF, discover how materials and physical forces behave differently at the nanoscale.
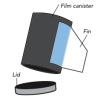
Pop Rockets
Source Institutions
In this activity, learners make film canister rocket ships. A fin pattern is glued onto the outside of the canister, and fuel (water and half an antacid tablet) is mixed inside the canister.

Temperature Affects Dissolving
Source Institutions
Learners design their own experiment to compare how well cocoa mix dissolves in cold and hot water. They will see that cocoa mix dissolves much better in hot water. Adult supervision recommended.

Changing the Density of an Object: Adding Material
Source Institutions
Learners see that a can of regular cola sinks while a can of diet cola floats. As a demonstration, bubble wrap is taped to the can of regular cola to make it float.
Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.

Look-alike Liquids
Source Institutions
Learners add drops of four liquids (water, alcohol, salt water, and detergent solution) to different surfaces and observe the liquids' behavior.
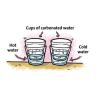
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.

A Dissolving Challenge
Source Institutions
In this activity, learners add objects and substances to carbonated water to discover that added objects increase the rate at which dissolved gas comes out of solution.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.

Polishing Pennies
Source Institutions
In this experiment, learners try different liquids to see which ones clean pennies best. Liquids to try include water, lemon juice, cola, vinegar, and dishwashing detergent.

Newspaper Collage
Source Institutions
In this activity on page 3 of the PDF, learners create a collage by using vinegar to transfer color pictures from a newspaper onto a piece of white paper.

A Feast for Yeast
Source Institutions
In this activity on page 6 of the PDF (Get Cooking With Chemistry), learners investigate yeast. Learners prepare an experiment to observe what yeast cells like to eat.
