Search Results
Showing results 241 to 260 of 590

Exploring Products: Nano Fabrics
Source Institutions
In this activity, learners explore how the application of nano-sized "whiskers" can protect clothing from stains.

The Ups and Downs of Thermometers
Source Institutions
In this activity, learners examine the parts of a thermometer. After placing a thermometer in hot and cold water, learners look at molecular model animations of the liquid in a thermometer.

Temperature Affects Dissolving
Source Institutions
Learners design their own experiment to compare how well cocoa mix dissolves in cold and hot water. They will see that cocoa mix dissolves much better in hot water. Adult supervision recommended.

Changing the Density of an Object: Adding Material
Source Institutions
Learners see that a can of regular cola sinks while a can of diet cola floats. As a demonstration, bubble wrap is taped to the can of regular cola to make it float.

Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.
Cooling the Mummy's Tomb
Source Institutions
In this activity, learners conduct an experiment to help Pharaoh design a better insulated tomb.

Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.

Imploding Pop Can
Source Institutions
In this dramatic activity/demonstration about phase change and condensation, learners place an aluminum can filled with about two tablespoons of water on a stove burner.

See It to Believe It: Visual Discrimination
Source Institutions
In this activity (12th on the page), learners investigate their ability to discriminate (see) different colors.

Portable Potable Pressure
Source Institutions
In this activity, learners use plastic water bottles, wood, and water to build an inexpensive and portable tool to demonstrate one atmosphere of pressure at sea level.

Water Quality and pH Levels in Aquatic Ecosystems
Source Institutions
In this fun and in depth hands-on experiment, learners test various liquid samples (distilled water, lemon juice, vinegar, and baking soda mixed with water) to determine their pH levels and identify e

Forgotten Genius
Source Institutions
This series of chemistry stations is designed to accompany the PBS documentary about African-American chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or p

Look-alike Liquids
Source Institutions
Learners add drops of four liquids (water, alcohol, salt water, and detergent solution) to different surfaces and observe the liquids' behavior.

M&M's in Different Temperatures
Source Institutions
Learners design their own experiment to investigate whether the temperature of the surrounding water affects the rate at which the colored coating dissolves from an M&M.

Loony Balloons
Source Institutions
In this activity, learners investigate how changing the center of gravity of a balloon affects how it travels. Learners fill a balloon with a little bit of water and insert into an empty balloon.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Buoyancy Bulls-Eye
Source Institutions
In this hands-on activity, learners will construct a scuba diver that can float in order to explore how sea creatures stay neutrally buoyant in the ocean and to see what kinds of forces might be influ
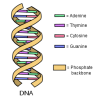
Close, Closer, Closest
Source Institutions
In this activity, learners perform an experiment that models a chromatography-like process called electrophoresis, a process used to analyze DNA.

DNA Extraction: Look at your genes!
Source Institutions
Extract your DNA from your very own cells! First, learners swish salt water in their mouth to collect cheek cells and spit the water into a glass.

Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).
