Search Results
Showing results 1 to 20 of 47

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Frozen Sculptures
Source Institutions
In this activity, learners use objects they find on a nature and water to make creative frozen sculptures.
Floating Paperclip and Other Surface Tension Experiments
Source Institutions
In this activity, learners experiment with surface tension using everyday household items such as strawberry baskets, paperclips, liquid dish soap, and pepper.

Convection Current
Source Institutions
In this activity, learners make their own heat waves in an aquarium.

Anti-Gravity Cups
Source Institutions
In this activity, learners will use simple materials to explore centripetal force and variables by swinging a cup of water without having the water spill out.

Magic Sand: Nanosurfaces
Source Institutions
This is an activity/demo in which learners are exposed to the difference bewteen hydrophobic surfaces (water repelling) and hydrophilic surfaces (water loving).

Freezing Lakes
Source Institutions
In some parts of the world, lakes freeze during winter. In this activity learners will explore water’s unique properties of freezing and melting, and how these relate to density and temperature.

Ice Fishing
Source Institutions
In this activity, learners will use string and salt to lift an ice cube out of a glass of water. Salt depresses the freezing point of water, allowing it to melt around the string and refreeze.

Indicating Electrolysis
Source Institutions
Electrolysis is the breakdown of water into hydrogen and oxygen. This Exploratorium activity allows learners to visualize the process with an acid-based indicator.

Radial Chromatography
Source Institutions
How many colors make black? Gather as many water soluble black markers as you can find.

Weightless Water
Source Institutions
In this physics activity (page 5 of the PDF), learners will witness the effects of free fall by observing falling water, and will gain a better understanding of the concept of weightlessness.

Tie Dye Secret Messages
Source Institutions
In this activity, learners will write a secret message that only their friends will be able to read.

Divers
Source Institutions
Learners experiment with a 2-liter plastic bottle containing water and four “divers." The divers consist of open, transparent containers with the opening points downward.

Mystery Sand
Source Institutions
In this activity, learners play with surprising sand that doesn’t get wet! Learners explore how water behaves differently when it comes in contact with "magic sand" and regular sand.

Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.
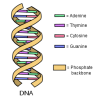
Close, Closer, Closest
Source Institutions
In this activity, learners perform an experiment that models a chromatography-like process called electrophoresis, a process used to analyze DNA.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

Got Gas?
Source Institutions
Create gas with a glass of water, some wire, conductors and a battery! You will be separating water (H2O) into oxygen and hydrogen.

Lotus Leaf Effect
Source Institutions
This is a demonstration about how nature inspires nanotechnology. It is easily adapted into a hands-on activity for an individual or groups.

Test Density with a Supersaturated Solution
Source Institutions
Learners create three solutions with different levels of salinity. They compare the density of these solutions by coloring them and layering them in a clear plastic cup and in a soda bottle.
