Search Results
Showing results 21 to 40 of 82

Handwashing Laboratory Activities: Bowl Technique
Source Institutions
In this lab (Activity #2 on page), learners compare bacteria growth on two petri dishes containing nutrient agar. Learners touch the doors, faucets, etc.

Fireworks in a Glass
Source Institutions
In this activity, learners use water, oil, and food coloring to observe a chemical reaction that creates a shower of colors inside of a glass.

Tie Dye Secret Messages
Source Institutions
In this activity, learners will write a secret message that only their friends will be able to read.
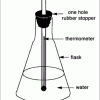
Using Solar Energy
Source Institutions
In this activity, learners discover how solar energy can be used to heat water.
Finding the Right Crater
Source Institutions
This quick demonstration (on page 11 of PDF) allows learners to understand why scientists think water ice could remain frozen in always-dark craters at the poles of the Moon.

Be A Pasta Food Scientist
Source Institutions
In this activity, learners of all ages can become food scientists by experimenting with flour and water to make basic pasta.

Egg Drop
Source Institutions
Perform this classic inertia demonstration to illustrate the transfer of potential energy to kinetic energy.

Inflate-a-mole
Source Institutions
In this activity, learners conduct an experiment to find the volume of one mole of gas. Learners capture sublimated gas from dry ice in a ziploc bag and use water displacement to measure its volume.

Measure the Pressure: The "Wet" Barometer
Source Institutions
In this activity, learners use simple items to construct a device for indicating air pressure changes.

Water Molds (Oomycetes)
Source Institutions
In this laboratory activity, learners use a simple procedure to bait oomycetes from water and/ or soil and then examine these fungus-like organisms with the microscope to see how they look.
Without An Ark: The Effects of Storms and Floods
Source Institutions
April showers bring May flowers, but what do coastal storms bring?

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Portable Potable Pressure
Source Institutions
In this activity, learners use plastic water bottles, wood, and water to build an inexpensive and portable tool to demonstrate one atmosphere of pressure at sea level.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.

Test Density with a Supersaturated Solution
Source Institutions
Learners create three solutions with different levels of salinity. They compare the density of these solutions by coloring them and layering them in a clear plastic cup and in a soda bottle.
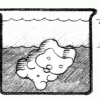
Gummy Growth
Source Institutions
In this activity related to Archimedes' Principle, learners use water displacement to compare the volume of an expanded gummy bear with a gummy bear in its original condition.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

A Crayon Rock Cycle- Metamorphic
Source Institutions
This is part 2 of the three-part "Crayon Rock Cycle" activity and must be done after part 1: Sedimentary Rocks. In this activity, learners explore how metamorphic rocks form.
