Search Results
Showing results 21 to 35 of 35

Burning Issues
Source Institutions
Learners use a candle to investigate the products of combustion. When a glass rod is held over a lit candle, the candle flame deposits carbon on the rod.

Rocket Science
Source Institutions
Learners create a small explosion by collecting hydrogen and oxygen gas together and squeezing them into a flame.

Balloon in a Flask
Source Institutions
Learners observe a flask with a balloon attached over the mouth and inverted inside the flask.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.
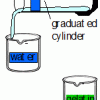
Natural Buffers
Source Institutions
Learners use a universal indicator to test the amount of sodium hydroxide needed to change the pH of plain water compared with the amount needed to change the pH of gelatin.

Yeast Balloons: Can biochemistry blow up a balloon?
Source Institutions
Using yeast, sugar, and water, learners create a chemical reaction which produces carbon dioxide (CO2) gas inside a 2-liter bottle. They use this gas to inflate a balloon.
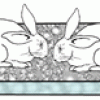
Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.
Good Vibrations
Source Institutions
This lesson (on pages 15-24 of PDF) explores how sound is caused by vibrating objects. It explains that we hear by feeling vibrations passing through the air.

Natural Indicators
Source Institutions
Learners combine different plant solutions -- made from fruits, vegetables, and flowers -- with equal amounts of vinegar (acid), water (neutral), and ammonia (base).
First Impressions
Source Institutions
Learners experiment with a commercial photo-sensitive paper (Sunprint® or NaturePrint® paper). They place opaque and clear objects on the paper and expose it to bright light, observing the results.

Electrolysis
Source Institutions
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water.

Currently Working
Source Institutions
Learners test solutions of water, sugar, salt, and hydrochloric acid for electrical conductivity. They immerse leads from a lighting device (a battery pack connected to an LED) into each solution.

Pot-in-Pot Refrigeration
Source Institutions
In this activity (on page 2 of PDF), learners create a low-tech refrigerator that requires no electricity to keep food from spoiling.

Cleaning with Dirt
Source Institutions
Learners build a filter from old soda bottles and dirt. They create polluted water, and pour it through their filter to clean it.
