Search Results
Showing results 1 to 20 of 45

Water Treatment
Source Institutions
Water treatment on a large scale enables the supply of clean drinking water to communities.

Water Underground
Source Institutions
Many people get water from a source deep underground, called groundwater.

Clear Water, Murky Water
Source Institutions
How do scientists measure how clear or murky water in a lake is? How does water clarity (clearness) affect what lives in the lake?

What's in the Water
Source Institutions
"What's in the Water" lets participants use tools to solve the mystery- what chemicals and compounds are in a sample of water?

The Scoop on Habitat
Source Institutions
Some aquatic organisms live in open water, while some live in soil at the bottom of a body of water.

Dye Detective
Source Institutions
Learners analyze mixtures of dyes using filter paper chromatography. They place spots of the different dyes at the bottom of a piece of filter paper, and hang the paper to touch the surface of water.

Window Under Water
Source Institutions
Glare from the sun and ripples from the wind can make it hard to see what's below the surface of a body of water.

Crocodiles
Source Institutions
Learners observe and compare the sizes of three toy “growing” crocodiles made from water-absorbent polymers. One is it its original state, dry, hard, and about 10cm long.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
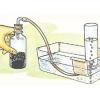
Pneumatic Trough
Source Institutions
In this activity, learners build a "pneumatic trough," a laboratory apparatus used for collecting pure gas samples over water.

Runaway Runoff
Source Institutions
When it rains, water can collect on top of and seep into the ground. Water can also run downhill, carrying soil and pollution with it.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.

Forgotten Genius
Source Institutions
This series of chemistry stations is designed to accompany the PBS documentary about African-American chemist "Percy Julian: Forgotten Genius." Each of the six stations features either a chemical or p
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).

Foam Peanuts
Source Institutions
Learners compare the properties and solubilities of Styrofoam (TM), ecofoam packing peanuts, and popcorn. First, the solubility of each substance is tested in water.
